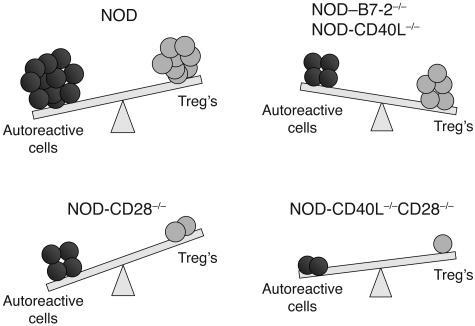
Figure 6.
Autoimmune diabetes is controlled by a costimulation-dependent balance of pathogenic and regulatory T cells. In NOD mice, which are genetically prone to autoimmunity, autoreactive T cells dominate Treg’s, leading to development of autoimmune diabetes. In the absence of B7-2 or CD40L, Treg’s are marginally reduced, whereas activation of autoreactive T cells is profoundly affected, resulting in the absence of diabetes. In contrast, in the absence of CD28 signals, the deficit in Treg’s is so severe that disease develops with accelerated kinetics and increased severity. Finally, in the absence of CD28 and CD40L signals, both diabetogenic T cells and Treg’s are affected, but the absence of CD28-dependent Treg’s appears dominant and results in insulitis and diabetes, thus bypassing the need for the two major costimulatory pathways in the development of the autoimmune process.