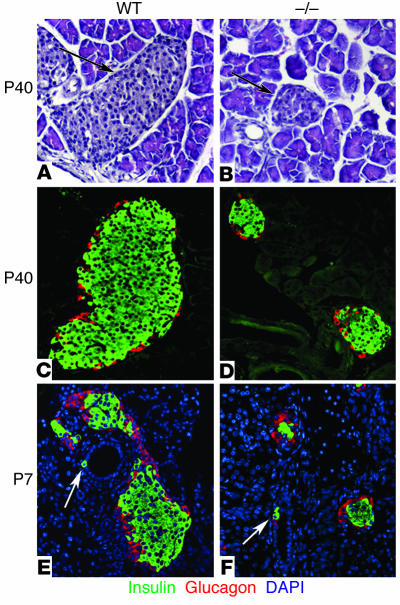
Figure 2.
Islet morphology and neogenesis of endocrine cells in WT and cyclin D2–/– mice. We stained pancreatic sections from 6-week-old WT and cyclin D2–/– (_/_) littermates with anti_glucagon and anti_insulin Ab’s. We estimated the islet size by measuring the longest axis of ten randomly selected islets that were at least ten cells wide. (A and B) H&E-stained pancreata from WT (A) and cyclin D2–/– (B) mice. The densely nucleated islets are indicated by arrows. (C) WT mice display a characteristic structure with α cells on the periphery and β cells at the core of the islet. (D) The cyclin D2–/– mice displayed dramatically smaller islets, although the morphology of α cells on the periphery and β cells at the core of the islet are preserved. The mean islet diameter of WT mice is 202 ± 13 μm versus 76.5 ± 4 μm for the cyclin D2–/– mice (P < 0.0001). (E and F) Pancreatic sections from P7 WT and cyclin D2–/– littermates stained with anti_insulin and anti_glucagon Ab’s. DAPI nuclear stain allows for the easy identification of ductal cells based on their characteristic morphology. Insulin-positive ductal cells in WT (E) and in cyclin D2–/– (F) littermates are shown (arrows). The frequency of hormone-positive ductal cells in the cyclin D2–/– mice is similar to that of WT littermates.