Figure 6.
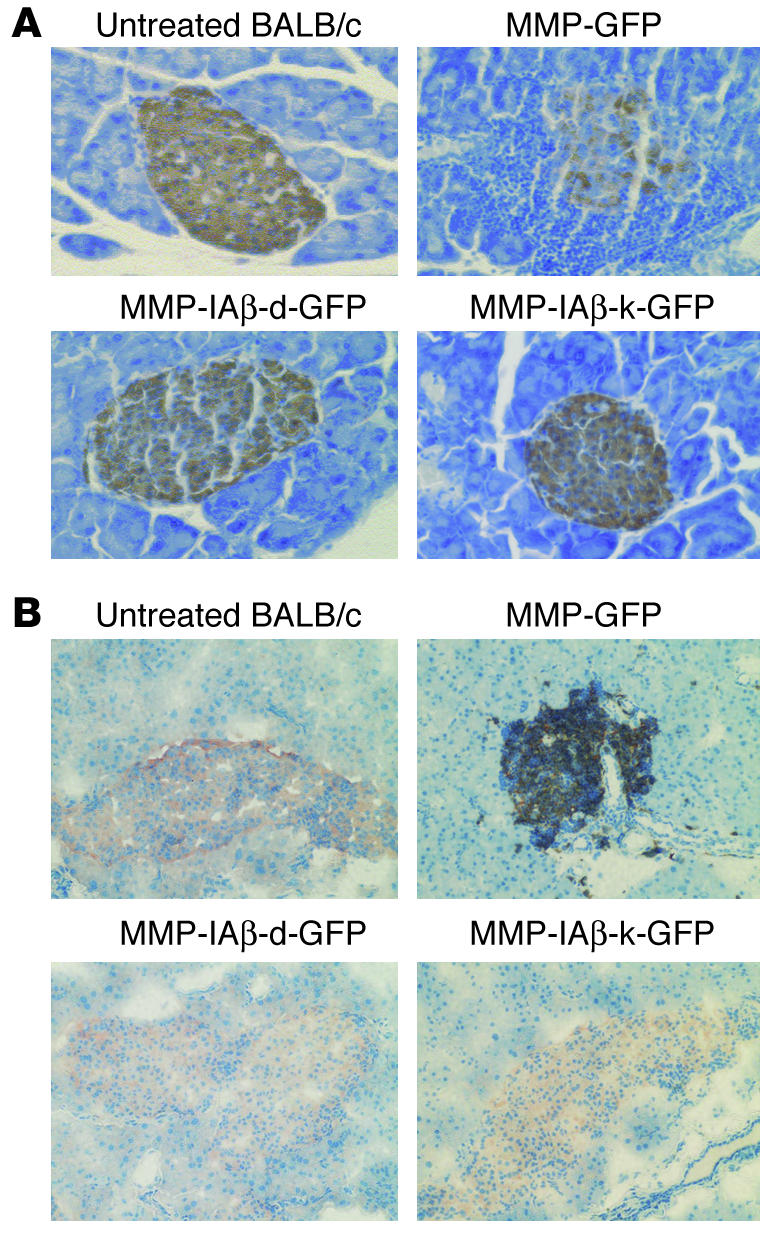
Prevention of insulitis in NOD mice reconstituted with MMP-IAβ-d-GFP_ or MMP-IAβ-k-GFP_transduced bone marrow. (A) NOD mice reconstituted with MMP-IAβ-d-GFP_, MMP-IAβ-k-GFP_, or MMP-GFP_transduced bone marrow were sacrificed 20 weeks after transplantation. Pancreata were then harvested, fixed, paraffin-embedded, sectioned, and stained with anti-insulin antibodies, and counterstained with hematoxylin. Islets from mice reconstituted with MMP-GFP_transduced bone marrow showed significant mononuclear cell infiltration, and decreased storage of insulin (brown staining, top right panel). In contrast, islets from mice reconstituted with either MMP-IAβ-d-GFP_transduced (bottom left) or MMP-IAβ-k-GFP_transduced bone marrow (bottom right) were free of cellular infiltration, and levels of insulin storage were comparable to those observed in tissue sections from control BALB/c pancreas (top left). Shown are representative sections from 3 independent experiments. (B) Cells infiltrating NOD islets are predominantly CD3+. NOD mice reconstituted with MMP-IAβ-d-GFP_, MMP-IAβ-k-GFP_, or MMP-GFP_transduced bone marrow were sacrificed 20 weeks after bone marrow transplantation. Pancreata were snap-frozen, sectioned, fixed, and stained with antibodies specific for insulin (red) and CD3 (black), and then counterstained with hematoxylin (blue). Pancreata from NOD mice reconstituted with MMP-GFP_transduced bone marrow (top right panel) show peri-islet infiltration of CD3+ cells, and little insulin staining. In contrast, pancreata from mice reconstituted with either MMP-IAβ-d-GFP_transduced (bottom left) or MMP-IAβ-k-GFP_transduced bone marrow (bottom right) had no visible cellular infiltrate, and insulin staining was comparable to that observed in control BALB/c pancreata (top left). Shown are representative sections from 3 independent experiments.
