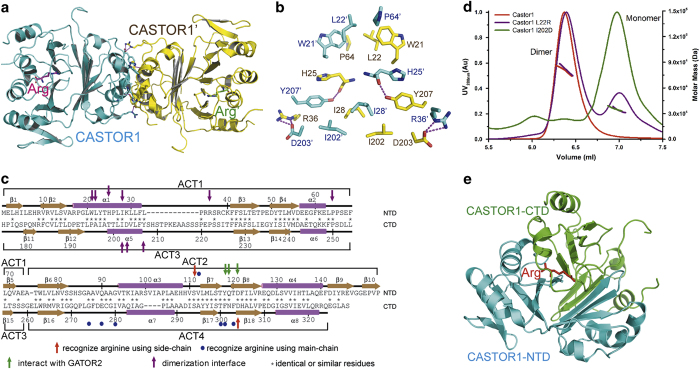
Figure 1.
Structure of human CASTOR1. (a) Overall structure of the human CASTOR1 dimer. The two CASTOR1 protomers are colored in cyan and yellow, respectively; and the two bound arginine molecules are colored in magenta and green, respectively. (b) The dimerization interface of CASTOR1. The residues belonging to the two CASTOR1 protomers are colored in cyan and yellow, respectively. Hydrogen bonds are shown as magenta dashed lines. (c) Residues of the NTD and the CTD of CASTOR1 are aligned, with their secondary structures shown. Residues recognizing arginine using side-chains or main-chains are marked by red arrows or blue circles, respectively; while residues interacting with GATOR2 and mediating CASTOR1 dimerization are indicated by green arrows and purple arrows, respectively. (d) Mutation of L22R or I202D disrupted dimerization of CASTOR1, as assayed by the SEC-MALS experiment. (e) Structure of a protomer of CASTOR1. The NTD and the CTD domains of CASTOR1 are colored in cyan and green, respectively. The bound arginine is shown in red.