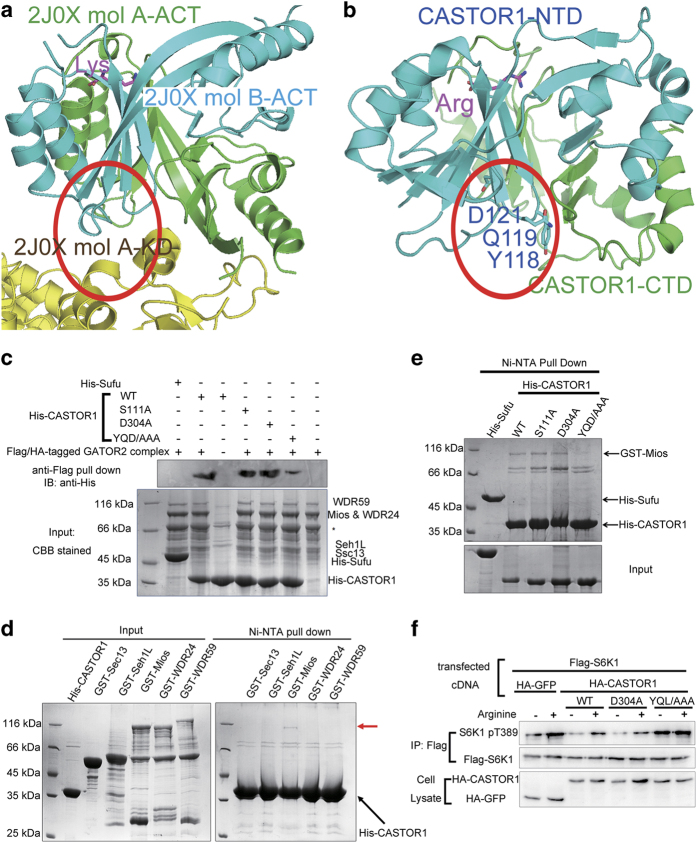
Figure 4.
A surface patch on CASTOR1-NTD on the opposite side of the arginine-binding site mediates its association with GATOR2. (a) The ACT domain of E. coli aspartate kinase (PDB code: 2J0X) employs a surface patch on the opposite side of its lysine-binding site to associate with its KD. In this structure, E. coli aspartate kinase exists as a dimer. The two protomers are denoted as mol A and mol B, respectively. (b) A surface patch on CASTOR1-NTD at a similar position as the E. coli aspartate kinase ACT domain’s binding site for its KD domain was identified to mediate its association with GATOR2. (c) Mutation of Y118A/Q119A/D121A on CASTOR1 decreased its interaction with the purified GATOR2 complex. His-tagged Suppressor of Fused (Sufu) protein served as a control. (d) Out of the five subunits of GATOR2, only Mios exhibited direct physical interaction with CASTOR1, as assayed by the Ni2+-NTA pull-down experiment using purified proteins. (e) Mutation of Y118A/Q119A/D121A on CASTOR1 almost abrogated its interaction with the purified Mios protein, a component of the GATOR2 complex. His-tagged Sufu served as a control. (f) Mutation of Y118A/Q119A/D121A abolished CASTOR1’s ability to inhibit GATOR2, and the downstream mTORC1 kinase phosphorylated S6K1 even in the absence of arginine.