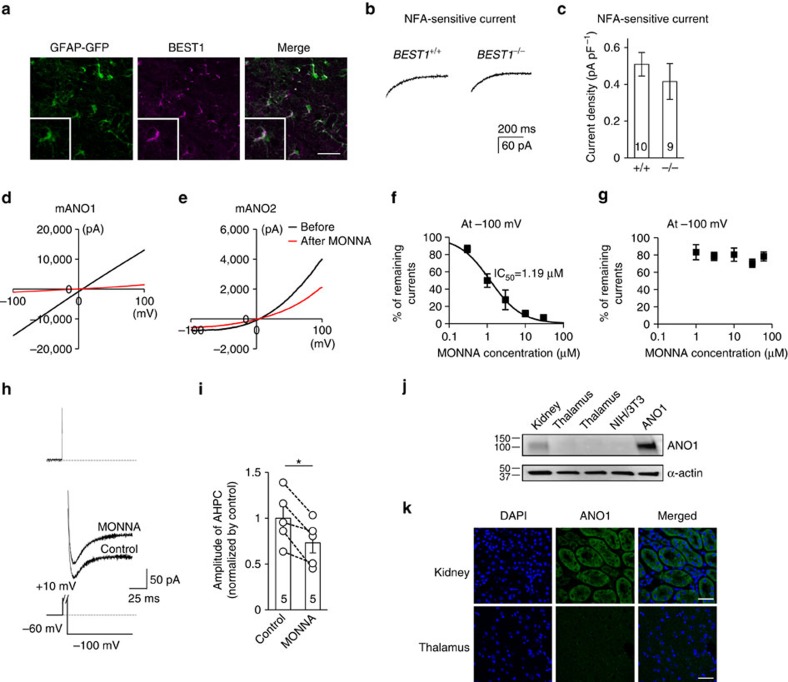
Figure 5. Ca2+-activated Cl− channels in the thalamus.
(a) Immunostaining showed that the BEST1 channel (purple) was expressed in the thalamus and co-localized with the astrocytic marker GFAP (green; scale bar, 40 μm). (b) Representative trace of an NFA-sensitive current was obtained by subtracting the tail current in the presence of TTX and apamin from the tail current in the presence of TTX, apamin and NFA in wild-type (left trace) and BEST1−/− (right trace) TC neurons. (c) Quantification of NFA-sensitive currents revealed that there were no differences between the NFA-sensitive currents of wild type (n=10 from six mice) and BEST−/− (n=9 from seven mice) TC neurons (P=0.40, t-test). (d,e) mANO1-IRES-EGFP or mANO2-EGFP-N1 was transfected into HEK293T cells. mANO1 and mANO2 currents were obtained by initially holding at 0 mV and then applying a 500 ms voltage ramp from −100 to +100 mV. Under whole-cell configuration, cells were treated with 30 μM MONNA and monitored until currents were blocked and stabilized. (f) Concentration-response curve at −100 mV indicated that the mANO1 current was almost completely abolished at high doses of MONNA (n=6; 0.3, 1, 3, 10 and 30 μM shown); the IC50 was 1.19 μM. (g) Concentration-response curve at −100 mV indicated that the mANO2 current remained largely intact even at high doses of MONNA (n=8; 1, 3, 10, 30 and 60 μM shown). (h) A representative trace of AHP-induced current from a cell before and during 30-μM MONNA application for 20 min. The protocol to generate AHP-induced currents is described in the lower panel. The dotted line above indicates the baseline level. (i) Summary bar graph showing the effect of MONNA on the amplitude of AHP currents (n=5 from three mice; *P=0.015, paired t-test). (j) Western blotting data indicated that ANO1 channels were not expressed in the thalamus. (k) Immunostaining data showed that ANO1 (green) signal in the thalamus is very weak compared with that in kidney tissue, which was used as a positive control (scale bar, 20 μm). Error bars represent mean±s.e.