Abstract
Vascular endothelial growth factor (VEGF) and bone morphogenetic proteins (BMPs), as key mediators in angiogenesis and osteogenesis, are used in a combined delivery manner as a novel strategy in bone tissue engineering. VEGF has the potential to enhance BMPs induced bone formation. Both gene delivery and material-based delivery systems were incorporated in previous studies to investigate the synergistic effects of VEGF and BMPs. However, their results were controversial due to variation of methods incorporated in different studies. Factors influencing the synergistic effects of VEGF on BMPs induced bone formation were identified and analyzed in this review to reduce confusion on this issue. The potential mechanisms and directions of future studies were also proposed here. Further investigating mechanisms of the synergistic effects and optimizing these influencing factors will help to generate more effective bone regeneration.
1. Introduction
Globally, approximately 15 million fractures are reported per year [1, 2], with 5–10% nonunion rate [1, 3, 4]. In addition, other diseases including tumors, infections, and degenerative diseases may also lead to bone defect. Bone is the second most commonly transplanted tissue, preceded only by blood transfusion [1]. Bone grafts performed in the United States alone are approximately 1.6 million per year, bringing huge medical and economic burden [2]. A variety of strategies have been developed to repair the diseased or defected bone. Autografts, which can provide desired growth factors, cells, and even microcirculation system, have always been regarded as the gold standard [3, 5, 6]. Unfortunately, autografts are restricted by some disadvantages, such as limited donor availability and donor-site morbidity [3, 6]. Allografts and xenografts as alternatives are limited by the potential disease transmission and immune rejection [3, 6, 7]. Tissue engineering is generally considered as a promising technique to overcome the disadvantages of traditional therapies.
A major objective of bone tissue engineering is to get effective bone regeneration, which is related to successful osteoinduction. Osteogenic cytokines, such as bone morphogenetic proteins (BMPs) and platelet-derived growth factor (PDGF), are the ideal candidates to enhance the osteoinduction [8, 9]. BMPs, identified and named by Urist [10, 11], belong to transforming growth factors β (TGF-β) superfamily. The osteogenic ability of BMPs has been well-documented in literatures [12]. Among them, BMP2, BMP4, BMP6, BMP7, and BMP9 possess osteogenic properties [12, 13]. Furthermore, rhBMP2 and rhBMP7 have been approved by FDA (US Food and Drug Administration) for specific clinical applications [12, 14]. However, in the last few years, some studies showed that desired clinical results could not been obtained by using BMPs alone. Moreover, complications caused by high-dose application were worrisome [3, 15–18].
Blood supply is arguably the largest challenge for any tissue engineering [25, 26]. Within the body, the effective diffusion distance of oxygen and nutrients is no more than 200 μm from the nearest capillary [25–27]. Bone is a highly vascularized tissue. Reconstructing local microcirculation is prerequisite for effective bone regeneration [28]. Inhibiting angiogenesis will reduce bone formation [29–31], while promoting angiogenesis can enhance bone regeneration [32]. Angiogenesis is regulated by several angiogenic factors, such as fibroblast growth factors (FGFs), transforming growth factor-α/β (TGF-α/β), PDGF, and notably vascular endothelial growth factor (VEGF) [15, 33–37]. Gerber et al. [29] investigated the role of VEGF in angiogenesis and bone formation. Their results shown that vascular invasion and bone formation were both suppressed by inhibiting VEGF in 24-day-old mice [29]. It was also revealed that application of VEGF-specific antagonist (soluble Flt1) could inhibit the bone regeneration induced by BMP4 and BMP2 [19, 20].
The undesirable outcomes of using BMPs alone and the importance of blood supply inspire tissue engineering scientists to explore the combined application of osteogenic and angiogenic factors [15, 38–40]. One of the most studied directions is the codelivery of BMPs and VEGF. The addition of VEGF is expected to enhance bone formation and reduce the amount of BMPs used.
2. The Combined Application of VEGF and BMPS
2.1. The Role of VEGF on BMPs Induced Bone Formation
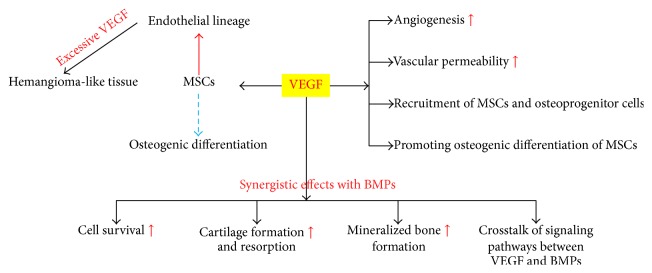
As a key mediator of angiogenesis [15, 38], VEGF also has direct and indirect effects on bone formation [7, 43]. VEGF may increase vascular permeability after promoting local angiogenesis [43, 49]. This will facilitate the recruitment of mesenchymal stem cells (MSCs) and osteoprogenitor cells to indirectly enhance the ability of bone regeneration [7, 43]. VEGF can also directly attract MSCs and promote their osteogenic differentiation [7, 43]. Enhanced neovascularization and bone regeneration were induced by the controlled release of VEGF in the study of Kaigler et al. [32]. After blocking VEGF, angiogenesis and osteogenesis were both inhibited [19, 20]. In addition to increasing angiogenesis and recruitment of MSCs, VEGF can act synergistically with BMPs to enhance cell survival, cartilage formation and resorption, and mineralized bone formation [19, 20]. Recently, the cross-talk of signaling pathways between VEGF and BMPs has gained growing attention [15, 50, 51]. Studies indicated that the synergistic effects of VEGF on BMPs induced bone formation were not only due to the increased angiogenesis (Figure 1). After the activation of VEGF signaling, the response of MSCs to BMP6 was significantly enhanced both in vitro and in vivo [15, 23, 51]. When treated with VEGF and BMP6, the expression of osteogenic genes including ALP, Dlx5, and osterix was significantly upregulated [50]. Furthermore, BMP-nonresponsive osteoprogenitor cells responded well to the costimulation of VEGF and BMP6 [51]. However, the accurate mechanisms are still unknown.
Figure 1.
The role of VEGF on BMPs induced bone formation.
2.2. Controversy on the Synergistic Effects between VEGF and BMPs
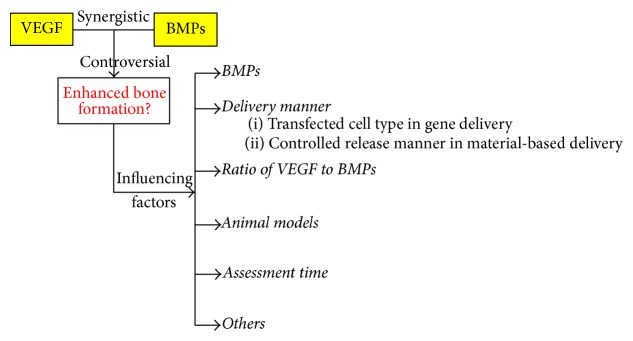
Although many studies have focused on this issue, whether VEGF can enhance BMPs induced bone formation in vivo is still very controversial [46]. The variation of influencing factors in different studies has led to completely opposite results, bringing much confusion on this issue. Based on literatures in this field, several important factors (Figure 2), which can significantly influence the synergistic effects between VEGF and BMPs, were identified and analyzed in the present review. These factors could partly explain the variations in the results of different studies and provide important information for future studies to generate more effective bone regeneration.
Figure 2.
Factors influencing the synergistic effects between VEGF and BMPs.
3. Influencing Factors
3.1. BMPs
Among BMP family, BMP2, BMP4, BMP6, BMP7, and BMP9 possess osteogenic properties [12, 13, 52–54]. They may share some properties in osteoinductive activity, but their interactions with VEGF are distinct. Synergistic effect of BMP4 with VEGF on bone formation is quite different from that of BMP2. Osteogenic effect of BMP4 was significantly affected by exogenous VEGF and it was more sensitive to the ratio of VEGF to BMP4 [19, 20]. High ratio of VEGF/BMP4 was obviously detrimental to the mineralized bone formation [19]. However, BMP2 could induced well-formed mineralized bone under high ratio of VEGF/BMP2, although the amount of bone formation also decreased compared to the group with lower ratio of VEGF/BMP2 [20]. The reason why BMP2 is less sensitive to VEGF remains unclear. A possible explanation is that BMP2 itself possesses angiogenic activity, leading to a decreased reaction to VEGF [20].
3.2. Delivery Manner of VEGF and BMPs
Traditional administration of growth factors is limited by their relatively short half-lives and potential side effects [55–57]. To overcome these disadvantages, gene delivery [6, 58–63] and material-based delivery system [64–70] have been developed in tissue engineering. There are mainly two strategies used in the codelivery of VEGF and BMPs. One of them is the expression of transgenes [19, 71–75] and the other is controlled release of growth factors from specific materials [38, 67, 76–78]. Transfected cell types in gene delivery and controlled release manners in material-based delivery can obviously influence the synergistic effects of VEGF and BMPs.
3.2.1. Transfected Cell Types in Gene Delivery
For transgenes therapy, plasmid, virus, and transfected cells are usually used as vectors or carriers for sustained expression of VEGF and/or BMPs [19–24, 79, 80] (Table 1). When transfected cells are transplanted in vivo, the synergistic effects of VEGF on BMPs induced bone formation are cell-type dependent. Peng et al. [19] transfected muscle-derived stem cells (MDSCs) to express VEGF or BMP4. Combined transplantation of VEGF- and BMP4-expressing cells resulted in significantly more bone formation compared to transplantation of BMP4-expressing cells alone [19]. Human periosteum-derived cells, osteoprogenitor cells, and bone marrow stromal cells (BMSCs) have been also proven to be effective carriers to achieve the synergistic effects between VEGF and BMPs [21, 23, 79]. However, when C2C12 cells (mouse myoblasts) and NIH/3T3 cells (mouse fibroblasts) were transfected to express BMP4 or VEGF + BMP4, VEGF inhibited the calcification of cells in vitro and exhibited a detrimental effect on bone formation in vivo [22].
Table 1.
Gene delivery of VEGF and BMPs.
| Authors | BMPs | Vectors (cell-type) |
Models | Expression in combination group | Ratio (VEGF/BMP) |
Bone formation in combined delivery group |
|---|---|---|---|---|---|---|
| Peng et al. 2002 [19] | BMP4 | Retroviral vectors (MDSCs) Cell-expressing BMP4 or VEGF |
Mice Intramuscular (4 weeks) Calvarial defect (3/6 weeks) Calvarial defect (3 weeks) |
BMP4: 115 ± 20 ng/106 cells/24 h VEGF: 214 ± 35 ng/106 cells/24 h (>4 weeks) |
Cell ratio: 1 : 5 1 : 5 1 : 5 (group 1, G1) 1 : 1 (group 2, G2) 5 : 1 (group 3, G3) |
Enhanced (compared to BMP4 alone) Enhanced (compared to BMP4 alone) Increased (compared to G3) Increased (compared to G3) Decreased (compared to G1/2) [19] |
|
| ||||||
| Peng et al. 2005 [20] | BMP2 | Retroviral vectors (MDSCs) Cell-expressing BMP2 or VEGF |
Mice Intramuscular (4 weeks) Calvarial defect (3/6 weeks) Calvarial defect (3 weeks) |
BMP2: 250 ng/106 cells/24 h VEGF: 200 ng/106 cells/24 h (>4 weeks) |
Cell ratio: 1 : 5 1 : 5 1 : 5 (group 1, G1) 1 : 1 (group 2, G2) 5 : 1 (group 3, G3) |
Enhanced (compared to BMP2 alone) Enhanced (compared to BMP2 alone) Increased (compared to G3) Increased (compared to G3) Decreased (compared to G1/2) [20] |
|
| ||||||
| Samee et al. 2008 [21] | BMP2 | Plasmid vectors (human periosteal cells) Cell-expressing BMP2 and/or VEGF |
Mice Intramuscular (4/8 weeks) |
BMP2: ~600–950 ng/106 cells/12–48 h VEGF: ~1.5–7.5 ng/106 cells/12–48 h (within 5 weeks) |
Plasmid ratio in cotransfection: 1 : 1 |
Enhanced at 4 weeks (compared to BMP2 alone) No significant difference at 8 weeks (compared to BMP2 alone) [21] |
|
| ||||||
| Li et al. 2009 [22] | BMP4 | Retroviral vectors (C2C12 and NIH/3T3 cells) Cell-expressing BMP4 and/or VEGF |
Mice Intramuscular (3-5weeks) |
C2C12: BMP4: 105 ± 10 ng/106 cells/24 h, VEGF: 152 ± 20 ng/106 cells/24 h NIH/3T3: BMP4: 98 ± 5 ng/106 cells/24 h VEGF: 128 ± 12 ng/106 cells/24 h (Retroviral vectors ratio 1 : 1) |
Retroviral vectors ratio in cotransfection: 1 : 1 1 : 5 1 : 50 |
Inhibited (compared to BMP4 alone) More detrimental effect Detrimental effect Less detrimental effect (compared to BMP4 alone) [22] |
|
| ||||||
| Cui et al. 2010 [23] | BMP6 | Plasmid vectors (cloned mouse osteoprogenitor cells) Cell-expressing BMP6 and/or VEGF |
Mice Subcutaneous (2/3 weeks) |
BMP6: ~1.8–18 ng/106 cells/24 h VEGF: ~0.12–12 ng/106 cells/24 h (within 2 weeks) |
VEGF/BMP6 gene (1 : 1) incorporated in plasmid constructs |
Enhanced (compared to BMP6 alone) [23] |
|
| ||||||
| Xiao et al. 2011 [24] | BMP2 |
Adenoviruse vectors (rabbit bone marrow stromal cells) Cell-expressing BMP2 or VEGF |
Rabbit Orbital defects (4/8/16 weeks) |
BMP2: ~300 pg/106 cells/24 h VEGF: ~300 pg/106 cells/24 h (within 8 weeks) |
Cell ratio: 1 : 4 (VEGF/BMP ratio 1 : 1) |
Enhanced (compared to BMP2 alone) [24] |
MDSCs = mouse muscle-derived stem cells, C2C12 cells = mouse myoblasts, and NIH/3T3 cells = mouse fibroblasts.
3.2.2. Controlled Release Manners in Material-Based Delivery
Studies have adopted different controlled release manners for delivering VEGF and BMPs to investigate their synergistic effects [7, 13, 41–48, 81–83] (Table 2). Biomaterials, such as gelatin, chitosan, collagen and poly (lactic-co-glycolic acid) (PLGA), can serve as carriers to release growth factors in a sustained manner in vivo [38, 84–87]. The controlled drug delivery system can be incorporated into porous materials to form a hybrid bone substitute scaffold, which can fill bone defect and induce effective bone repair. During normal bone regeneration, the expression of VEGF is upregulated in the early days and peaks around day 5–10 [43, 88–90], while normal expression of BMPs peaks at day 21 and thereafter [43, 91, 92]. In order to achieve the sequential release of growth factors, Kempen et al. [43] adopted PLGA microspheres and poly (propylene) scaffold as a sustained release system of BMP2 and used gelatin hydrogel as a fast release system of VEGF. The in vivo release profiles of VEGF showed an initial burst release in the first 3 days (89.9 ± 2.9% at the ectopic site). The remaining VEGF exhibited a sustained release over 35 days at a low level. The release of BMP2 was sustained over 56 days [43]. Ectopic bone formation was significantly enhanced by the combined application of VEGF and BMP2 compared to that of BMP2 alone [43]. However, some other authors supported more sustained delivery (nonfast release) of VEGF [7, 32]. The effect of fast release of VEGF is still controversial. The vascular network induced by VEGF alone is immature [26, 93–95]. If its concentration falls too low before the formation of mature vascular network, the unstable vascular network may be remodeled or trimmed [42, 95, 96]. This is also supported by the results of previous studies that synergistic effect of VEGF and BMP2 was only presented at 4 weeks in vivo, while being absent at 12 weeks [42]. In both groups of VEGF alone and VEGF + BMP2, a decrease of vascular density was observed at 12 weeks compared to that at 4 weeks [46]. In fact the effective delivery manner of VEGF in the study of Kempen et al. [43] is more like a composite model: burst release in the early stage and sustained delivery at a low level in later stage. The optimal delivery manner of VEGF needs to be further studied. In addition, combining other angiogenic factors, such as PDGF, to facilitate the maturity and stability of neovascularization may be more effective [95].
Table 2.
Controlled release VEGF and BMPs.
| Authors | BMPs | Carrier | Models | Combination delivery | Ratio (VEGF/BMP) |
Bone formation in combined delivery group |
|---|---|---|---|---|---|---|
| Kakudo et al. 2006 [41] | BMP2 | Collagen | Rat Intramuscular (3 weeks) |
Simultaneous | 1 : 2 | Enhanced (compared to BMP2 alone) [41] |
|
| ||||||
| Patel et al. 2008 [42] | BMP2 | Gelatin | Rat Calvarial defect (4/12 weeks) |
Simultaneous | 6 : 1 | Enhanced at 4 weeks (compared to BMP2 alone) No significant difference at 12 weeks (compared to BMP2 alone) [42] |
|
| ||||||
| Kempen et al. 2009 [43] | BMP2 | PLGA-BMP2 (sustained release) Gelatin-VEGF (fast release) |
Rat Subcutaneous (8 weeks) Segmental femoral defect (8 weeks) |
Sequential | 1 : 3.3 | Ectopic: enhanced (compared to BMP2 alone) Orthotopic: no significant difference (compared to BMP2 alone) [43] |
|
| ||||||
| Young et al. 2009 [44] | BMP2 | Gelatin | Rat Calvarial defect (12 weeks) |
Simultaneous | 6/12/24 : 1 | No significant difference (compared to BMP2 alone) [44] |
|
| ||||||
| Roldán et al. 2010 [45] | BMP7 | BCP scaffold (growth factors injected in the scaffolds) | Mice Subcutaneous (12 weeks) |
Simultaneous | 2 : 5 | No significant difference (compared to BMP7 alone) [45] |
|
| ||||||
| Zhang et al. 2011 [7] | BMP2 | Silk hydrogels | Rabbits Sinus floor elevation model (4/12 weeks) |
Simultaneous |
2 : 3 | Enhanced (compared to BMP2 alone) [7] |
|
| ||||||
| Geuze et al. 2012 [13] | BMP2 | PLGA-VEGF/BMP2 (fast release) Gelatin-VEGF/BMP2 (sustained release) |
Dog Ectopic: intramuscular (9 weeks) Orthotopic site: ulnar defect (9 weeks) |
Sequential or simultaneous | 1 : 30 | No significant enhancement effect (compared to BMP2 alone) [13] |
|
| ||||||
| Hernández et al. 2012 [46] | BMP2 | PLGA | Rabbit Intramedullary femur defect (4/12 weeks) |
Simultaneous | 1 : 10/50 | Enhanced at 4 weeks (compared to BMP2 alone) No significant difference at 12 weeks (compared to BMP2 alone) [46] |
|
| ||||||
| Das et al. 2015 [47] | BMP6 | PLGA | Rat Mandibular defect (2/8/12 weeks) |
Simultaneous | 1 : 1 | Enhanced (compared to BMP6 alone) [47] |
|
| ||||||
| Lv et al. 2015 [48] | BMP2 | Fibrin glue (fast release) | Rabbit Femoral condyle defect (4 weeks) |
Simultaneous | 1 : 100 | No synergistic effect (compared to BMP2 alone) [48] |
Although some authors reported that fast release of BMP2 might induce more ectopic bone regeneration than its sustained release [13], more papers confirmed that the sustained delivery strategy would prolong its activity and reduce its potential side effects [7, 43, 97–101].
3.3. Ratio of VEGF to BMPs
The ratio of VEGF to BMPs has an obvious impact on their synergistic effects. Although the interactions between BMPs and VEGF are inconsistent among different kinds of BMPs, there is a similar trend that VEGF seems to be more effective at low ratio of VEGF/BMPs than at a high ratio [19, 20]. Peng et al. [19] have adopted the cotransplantation of VEGF- and BMP4-expressing muscle-derived stem cells at different ratios to study its relationship with bone formation. The amount of bone formation in groups with the ratios of VEGF/BMP4 at 1 : 5 and 1 : 1 was significantly larger compared to that in the group with a ratio of 5 : 1 [19]. The interactions of VEGF and BMPs are based on their influence on the function and differentiation of target cells. Under high ratio of VEGF/BMPs, excessive VEGF will push local MSCs towards an endothelial lineage, reducing the cells available for osteogenic differentiation [19, 44]. It was reported that high dose of VEGF might lead to hemangioma-like tissue formation [102, 103]. It was also suspected that high ratio of VEGF/BMPs may increase the recruitment and survival of osteoclasts, leading to excessive bone resorption [15, 104–106]. However, Peng et al. [19] disagreed with this. In their study, the markers of osteoclasts were similar in groups with low and high ratios of VEGF/BMP4 [19]. It is important to note that ratio of VEGF/BMPs reported in most studies is the ratio of total dose of growth factors [42, 44] or total amount of transfected cells used [19, 20]. However, what actually affects the bone formation is the ratio of released growth factors. As reported by Lohse et al. [83], continuous delivery of VEGF and BMP2 at a ratio approximately 1 could significantly increase the induced bone formation compared to that at a ratio ≤0.5. Future studies should further investigate the relationship between the ratio of released VEGF/BMPs and the amount of bone formation both in vitro and in vivo.
3.4. Animal Models
When other experimental conditions are controlled to be consistent, the synergistic effects of VEGF and BMPs vary among different animal models. In the same studies [13, 43], synergistic effects between VEGF and BMPs were only observed in ectopic models, while being absent in orthopaedic sites, indicating the synergistic effect was location-dependent. Facilitating the recruitment of MSCs is one of the mechanisms why VEGF can enhance bone formation elicited by BMPs. Nevertheless, in the bone defect site, periosteum and exposed marrow cavity can offer an abundant of MSCs [43]. Furthermore, local hematoma in orthopaedic site may serve as a source of endogenous angiogenic factors [48, 107–109]. The abundant source of MSCs and increased endogenous angiogenic factors may decrease the effect of exogenous VEGF. The synergistic effects between VEGF and BMPs are supposed to be more prominent in areas suffering from compromised circulation, such as ischemia model and old bone defect model.
3.5. Assessment Time
The synergistic effects of VEGF and BMPs might be observed in a short study period, while being absent in an extended period [42, 46]. The decrease of concentration of growth factors, such as VEGF, may partly explain this, as analyzed above. Another possibility proposed in this review is whether the application of exogenous growth factors will downregulate the secretion of endogenous VEGF and BMPs within a certain period. If so, after depletion of exogenous growth factors, the lack of endogenous growth factors will be detrimental to bone regeneration. Extending observation period and setting different time points should be helpful to get further understanding of the synergistic effects and to optimize the combination application strategies in the future studies.
3.6. Other Influencing Factors
In addition to the factors mentioned above, material carriers of the delivery system, methods used in assessment of bone formation, and the introduction of other growth factors or cells might also influence the evaluation of synergistic effects of VEGF and BMPs. Effective control of these related factors can help us to get further understanding of the mechanisms of the interactions between these two key growth factors in angiogenesis and osteogenesis.
4. Conclusions
The combined delivery of VEGF and BMPs is a novel and promising strategy in bone tissue engineering. VEGF can help to promote the construction of vascular network, to improve the local supply of oxygen and nutrients, to increase the recruitment and survival of MSCs, and to enhance the response of MSCs to BMPs. When they are used in a combined delivery manner in vivo, VEGF has the potential to synergistically enhance BMPs induced bone formation. Many studies have been conducted to investigate the effect of this approach. However, due to the variation of BMPs, carriers of growth factors, controlled release manners, growth factors ratio, models, and assessment time, their results are pretty controversial. These influencing factors were identified and analyzed in this review to avoid more confusion on this issue. Future studies should further investigate the mechanisms of their synergistic effects and optimize these influencing factors to generate more effective bone regeneration.
Acknowledgments
This work was supported by grants from Peking Union Medical College Youth Fund (no. 33320140019) and Peking Union Medical College graduate student innovation Fund (no. B2014001060).
Disclosure
Bo Li and Hai Wang are the first co-authors.
Competing Interests
The authors declare that they have no conflict of interests.
References
- 1.Liu Y., Lim J., Teoh S.-H. Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnology Advances. 2013;31(5):688–705. doi: 10.1016/j.biotechadv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe R. J., Mao J. Bone tissue engineering and regeneration: from discovery to the clinic—an overview. Tissue Engineering—Part B: Reviews. 2011;17(6):389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C.-H., Chang Y.-H., Lin S.-Y., Li K.-C., Hu Y.-C. Recent progresses in gene delivery-based bone tissue engineering. Biotechnology Advances. 2013;31(8):1695–1306. doi: 10.1016/j.biotechadv.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Salgado A. J., Coutinho O. P., Reis R. L. Bone tissue engineering: state of the art and future trends. Macromolecular Bioscience. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 5.Kneser U., Schaefer D. J., Polykandriotis E., Horch R. E. Tissue engineering of bone: the reconstructive surgeon's point of view. Journal of Cellular and Molecular Medicine. 2006;10(1):7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kofron M. D., Laurencin C. T. Bone tissue engineering by gene delivery. Advanced Drug Delivery Reviews. 2006;58(4):555–576. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W., Wang X., Wang S., et al. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials. 2011;32(35):9415–9424. doi: 10.1016/j.biomaterials.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman J. R., Daluiski A., Einhorn T. A. The role of growth factors in the repair of bone. Biology and clinical applications. Journal of Bone and Joint Surgery—Series A. 2002;84(6):1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman J. R., Daluiski A., Stevenson S., et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. Journal of Bone and Joint Surgery—Series A. 1999;81(7):905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Urist M. R. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 11.Urist M. R., Strates B. S. Bone morphogenetic protein. Journal of Dental Research. 1971;50(6):1392–1406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- 12.Bessa P. C., Casal M., Reis R. L. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) Journal of Tissue Engineering and Regenerative Medicine. 2008;2(1):1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 13.Geuze R. E., Theyse L. F. H., Kempen D. H. R., et al. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Engineering - Part A. 2012;18(19-20):2052–2062. doi: 10.1089/ten.tea.2011.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bessa P. C., Casal M., Reis R. L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) Journal of Tissue Engineering and Regenerative Medicine. 2008;2(2-3):81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 15.Cui Q., Dighe A. S., Irvine J. N., Jr. Combined angiogenic and osteogenic factor delivery for bone regenerative engineering. Current Pharmaceutical Design. 2013;19(19):3374–3383. doi: 10.2174/1381612811319190004. [DOI] [PubMed] [Google Scholar]
- 16.Mesfin A., Buchowski J. M., Zebala L. P., et al. High-dose rhBMP-2 for adults: major and minor complications: a study of 502 spine cases. Journal of Bone and Joint Surgery—Series A. 2013;95(17):1546–1553. doi: 10.2106/jbjs.l.01730. [DOI] [PubMed] [Google Scholar]
- 17.Fu R., Selph S., McDonagh M., et al. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Annals of Internal Medicine. 2013;158(12):890–902. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gothard D., Smith E. L., Kanczler J. M., et al. Tissue engineered bone using select growth factors: a comprehensive review of animal studies and clinical translation studies in man. European Cells and Materials. 2014;28:166–207. doi: 10.22203/ecm.v028a13. [DOI] [PubMed] [Google Scholar]
- 19.Peng H., Wright V., Usas A., et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. Journal of Clinical Investigation. 2002;110(6):751–759. doi: 10.1172/JCI200215153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng H., Usas A., Olshanski A., et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. Journal of Bone and Mineral Research. 2005;20(11):2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 21.Samee M., Kasugai S., Kondo H., Ohya K., Shimokawa H., Kuroda S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. Journal of Pharmacological Sciences. 2008;108(1):18–31. doi: 10.1254/jphs.08036fp. [DOI] [PubMed] [Google Scholar]
- 22.Li G., Corsi-Payne K., Zheng B., Usas A., Peng H., Huard J. The dose of growth factors influences the synergistic effect of vascular endothelial growth factor on bone morphogenetic protein 4-induced ectopic bone formation. Tissue Engineering—Part A. 2009;15(8):2123–2133. doi: 10.1089/ten.tea.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui F., Wang X., Liu X., Dighe A. S., Balian G., Cui Q. VEGF and BMP-6 enhance bone formation mediated by cloned mouse osteoprogenitor cells. Growth Factors. 2010;28(5):306–317. doi: 10.3109/08977194.2010.484423. [DOI] [PubMed] [Google Scholar]
- 24.Xiao C., Zhou H., Liu G., et al. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomedical Materials. 2011;6(1) doi: 10.1088/1748-6041/6/1/015013.015013 [DOI] [PubMed] [Google Scholar]
- 25.Lovett M., Lee K., Edwards A., Kaplan D. L. Vascularization strategies for tissue engineering. Tissue Engineering - Part B: Reviews. 2009;15(3):353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain R. K., Au P., Tam J., Duda D. G., Fukumura D. Engineering vascularized tissue. Nature Biotechnology. 2005;23(7):821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 27.Fidkowski C., Kaazempur-Mofrad M. R., Borenstein J., Vacanti J. P., Langer R., Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Engineering. 2005;11(1-2):302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 28.Saran U., Gemini Piperni S., Chatterjee S. Role of angiogenesis in bone repair. Archives of Biochemistry and Biophysics. 2014;561:109–117. doi: 10.1016/j.abb.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Gerber H.-P., Vu T. H., Ryan A. M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Medicine. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 30.Mori S., Yoshikawa H., Hashimoto J., et al. Antiangiogenic agent (TNP-470) inhibition of ectopic bone formation induced by bone morphogenetic protein-2. Bone. 1998;22(2):99–105. doi: 10.1016/S8756-3282(97)00248-2. [DOI] [PubMed] [Google Scholar]
- 31.Hausman M. R., Schaffler M. B., Majeska R. J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29(6):560–564. doi: 10.1016/S8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 32.Kaigler D., Wang Z., Horger K., Mooney D. J., Krebsbach P. H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. Journal of Bone and Mineral Research. 2006;21(5):735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 33.Zisch A. H., Lutolf M. P., Hubbell J. A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovascular Pathology. 2003;12(6):295–310. doi: 10.1016/S1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 34.Zisch A. H., Lutolf M. P., Ehrbar M., et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB Journal. 2003;17(15):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 35.Bauer S. M., Bauer R. J., Liu Z.-J., Chen H., Goldstein L., Velazquez O. C. Vascular endothelial growth factor-C promotes vasculogenesis, angiogenesis, and collagen constriction in three-dimensional collagen gels. Journal of Vascular Surgery. 2005;41(4):699–707. doi: 10.1016/j.jvs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Andrae J., Gallini R., Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes and Development. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perets A., Baruch Y., Weisbuch F., Shoshany G., Neufeld G., Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. Journal of Biomedical Materials Research—Part A. 2003;65(4):489–497. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.-H., Tabata Y. Dual-controlled release system of drugs for bone regeneration. Advanced Drug Delivery Reviews. 2015;94:28–40. doi: 10.1016/j.addr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Kanczler J. M., Oreffo R. O. C. Osteogenesis and angiogenesis: the potential for engineering bone. European Cells and Materials. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 40.Subbiah R., Hwang M. P., Van S. Y., et al. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Advanced Healthcare Materials. 2015;4(13):1982–1992. doi: 10.1002/adhm.201500341. [DOI] [PubMed] [Google Scholar]
- 41.Kakudo N., Kusumoto K., Wang Y. B., Iguchi Y., Ogawa Y. Immunolocalization of vascular endothelial growth factor on intramuscular ectopic osteoinduction by bone morphogenetic protein-2. Life Sciences. 2006;79(19):1847–1855. doi: 10.1016/j.lfs.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 42.Patel Z. S., Young S., Tabata Y., Jansen J. A., Wong M. E. K., Mikos A. G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kempen D. H. R., Lu L., Heijink A., et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Young S., Patel Z. S., Kretlow J. D., et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Engineering—Part A. 2009;15(9):2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roldán J. C., Detsch R., Schaefer S., et al. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. Journal of Cranio-Maxillofacial Surgery. 2010;38(6):423–430. doi: 10.1016/j.jcms.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Hernández A., Reyes R., Sánchez E., Rodríguez-Évora M., Delgado A., Évora C. In vivo osteogenic response to different ratios of BMP-2 and VEGF released from a biodegradable porous system. Journal of Biomedical Materials Research Part A. 2012;100(9):2382–2391. doi: 10.1002/jbm.a.34183. [DOI] [PubMed] [Google Scholar]
- 47.Das A., Fishero B. A., Christophel J. J., et al. Poly(lactic-co-glycolide) polymer constructs cross-linked with human BMP-6 and VEGF protein significantly enhance rat mandible defect repair. Cell and Tissue Research. 2015 doi: 10.1007/s00441-015-2301-x. [DOI] [PubMed] [Google Scholar]
- 48.Lv J., Xiu P., Tan J., Jia Z., Cai H., Liu Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: implantation of electron beam melting-fabricated porous Ti6Al4V scaffolds incorporating growth factor-doped fibrin glue. Biomedical Materials (Bristol) 2015;10(3) doi: 10.1088/1748-6041/10/3/035013.035013 [DOI] [PubMed] [Google Scholar]
- 49.Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. The FASEB Journal. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 50.Zhang Y., Madhu V., Dighe A. S., Irvine J. N., Jr., Cui Q. Osteogenic response of human adipose-derived stem cells to BMP-6, VEGF, and combined VEGF plus BMP-6 in vitro. Growth Factors. 2012;30(5):333–343. doi: 10.3109/08977194.2012.720574. [DOI] [PubMed] [Google Scholar]
- 51.Madhu V., Li C.-J., Dighe A. S., Balian G., Cui Q. BMP-non-responsive Sca1+CD73+CD44+ mouse bone marrow derived osteoprogenitor cells respond to combination of VEGF and BMP-6 to display enhanced osteoblastic differentiation and ectopic bone formation. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0103060.e103060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang Q., Sun M. H., Cheng H., et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Therapy. 2004;11(17):1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 53.Bostrom M. P. G., Lane J. M., Berberian W. S., et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. Journal of Orthopaedic Research. 1995;13(3):357–367. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- 54.Wei G., Jin Q., Giannobile W. V., Ma P. X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28(12):2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf M. T., Dearth C. L., Sonnenberg S. B., Loboa E. G., Badylak S. F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Advanced Drug Delivery Reviews. 2015;84:208–221. doi: 10.1016/j.addr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almubarak S., Nethercott H., Freeberg M., et al. Tissue engineering strategies for promoting vascularized bone regeneration. Bone. 2016;83:197–209. doi: 10.1016/j.bone.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell A. C., Briquez P. S., Hubbell J. A., Cochran J. R. Engineering growth factors for regenerative medicine applications. Acta Biomaterialia. 2016;30:1–12. doi: 10.1016/j.actbio.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pola E., Gao W., Zhou Y., et al. Efficient bone formation by gene transfer of human LIM mineralization protein-3. Gene Therapy. 2004;11(8):683–693. doi: 10.1038/sj.gt.3302207. [DOI] [PubMed] [Google Scholar]
- 59.Yang S., Wei D., Wang D., Phimphilai M., Krebsbach P. H., Franceschi R. T. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. Journal of Bone and Mineral Research. 2003;18(4):705–715. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peterson B., Zhang J., Iglesias R., et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Engineering. 2005;11(1-2):120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 61.Shen H.-C., Peng H., Usas A., Gearhart B., Fu F. H., Huard J. Structural and functional healing of critical-size segmental bone defects by transduced muscle-derived cells expressing BMP4. Journal of Gene Medicine. 2004;6(9):984–991. doi: 10.1002/jgm.588. [DOI] [PubMed] [Google Scholar]
- 62.Yang M., Ma Q.-J., Dang G. T., Ma K. T., Chen P., Zhou C.-Y. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005;7(3):273–281. doi: 10.1080/14653240510027244. [DOI] [PubMed] [Google Scholar]
- 63.Lee H.-L., Lee H. Y., Yun Y., et al. Hypoxia-specific, VEGF-expressing neural stem cell therapy for safe and effective treatment of neuropathic pain. Journal of Controlled Release. 2016;226:21–34. doi: 10.1016/j.jconrel.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 64.Su J., Xu H., Sun J., Gong X., Zhao H. Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration. International Journal of Molecular Sciences. 2013;14(6):12714–12728. doi: 10.3390/ijms140612714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lei L., Wang S., Wu H., et al. Optimization of release pattern of FGF-2 and BMP-2 for osteogenic differentiation of low-population density hMSCs. Journal of Biomedical Materials Research—Part A. 2015;103(1):252–261. doi: 10.1002/jbm.a.35168. [DOI] [PubMed] [Google Scholar]
- 66.Facca S., Cortez C., Mendoza-Palomares C., et al. Active multilayered capsules for in vivo bone formation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(8):3406–3411. doi: 10.1073/pnas.0908531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De la Riva B., Sánchez E., Hernández A., et al. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. Journal of Controlled Release. 2010;143(1):45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 68.Kim B. S., Kim J. S., Yang S. S., Kim H. W., Lim H. J., Lee J. Angiogenin-loaded fibrin/bone powder composite scaffold for vascularized bone regeneration. Biomaterials Research. 2015;19, article 18 doi: 10.1186/s40824-015-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Y., Zhu S., Luo E., Li J., Feng G., Hu J. Basic fibroblast growth factor suspended in Matrigel improves titanium implant fixation in ovariectomized rats. Journal of Controlled Release. 2009;139(1):15–21. doi: 10.1016/j.jconrel.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 70.Jung Y. J., Kim K., Heo J., et al. Induction of angiogenesis by matrigel coating of VEGF-loaded PEG/PCL-based hydrogel scaffolds for hbmsc transplantation. Molecules and Cells. 2015;38(7):663–668. doi: 10.14348/molcells.2015.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans C. H., Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nature Reviews Rheumatology. 2015;11(4):234–242. doi: 10.1038/nrrheum.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sieker J. T., Kunz M., Weißenberger M., et al. Direct bone morphogenetic protein 2 and Indian hedgehog gene transfer for articular cartilage repair using bone marrow coagulates. Osteoarthritis and Cartilage. 2015;23(3):433–442. doi: 10.1016/j.joca.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Wright V. J., Peng H., Usas A., et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Molecular Therapy. 2002;6(2):169–178. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 74.Zhu L., Chuanchang D., Wei L., Yilin C., Jiasheng D. Enhanced healing of goat femur-defect using BMP7 gene-modified BMSCs and load-bearing tissue-engineered bone. Journal of Orthopaedic Research. 2010;28(3):412–418. doi: 10.1002/jor.20973. [DOI] [PubMed] [Google Scholar]
- 75.Li R., Stewart D. J., Von Schroeder H. P., Mackinnon E. S., Schemitsch E. H. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. Journal of Orthopaedic Research. 2009;27(1):8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 76.Hélary C., Desimone M. F. Recent advances in biomaterials for tissue engineering and controlled drug delivery. Current Pharmaceutical Biotechnology. 2015;16(7):635–645. doi: 10.2174/138920101607150427112208. [DOI] [PubMed] [Google Scholar]
- 77.Kaito T., Myoui A., Takaoka K., et al. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials. 2005;26(1):73–79. doi: 10.1016/j.biomaterials.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Yonamine Y., Matsuyama T., Sonomura T., et al. Effectable application of vascular endothelial growth factor to critical sized rat calvaria defects. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2010;109(2):225–231. doi: 10.1016/j.tripleo.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y.-C., Kaigler D., Rice K. G., Krebsbach P. H., Mooney D. J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. Journal of Bone and Mineral Research. 2005;20(5):848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 80.Luo T., Zhang W., Shi B., Cheng X., Zhang Y. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clinical Oral Implants Research. 2012;23(4):467–473. doi: 10.1111/j.1600-0501.2011.02164.x. [DOI] [PubMed] [Google Scholar]
- 81.Ramazanoglu M., Lutz R., Ergun C., von Wilmowsky C., Nkenke E., Schlegel K. A. The effect of combined delivery of recombinant human bone morphogenetic protein-2 and recombinant human vascular endothelial growth factor 165 from biomimetic calcium-phosphate-coated implants on osseointegration. Clinical Oral Implants Research. 2011;22(12):1433–1439. doi: 10.1111/j.1600-0501.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 82.Schmitt C., Lutz R., Doering H., Lell M., Ratky J., Schlegel K. A. Bio-Oss® blocks combined with BMP-2 and VEGF for the regeneration of bony defects and vertical augmentation. Clinical Oral Implants Research. 2013;24(4):450–460. doi: 10.1111/j.1600-0501.2011.02351.x. [DOI] [PubMed] [Google Scholar]
- 83.Lohse N., Moser N., Backhaus S., Annen T., Epple M., Schliephake H. Continuous delivery of rhBMP2 and rhVEGF165 at a certain ratio enhances bone formation in mandibular defects over the delivery of rhBMP2 alone—An experimental study in rats. Journal of Controlled Release. 2015;220:201–209. doi: 10.1016/j.jconrel.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 84.Wang H., Wu G., Zhang J., et al. Osteogenic effect of controlled released rhBMP-2 in 3D printed porous hydroxyapatite scaffold. Colloids and Surfaces B: Biointerfaces. 2016;141:491–498. doi: 10.1016/j.colsurfb.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Ueda H., Hong L., Yamamoto M., et al. Use of collagen sponge incorporating transforming growth factor-β1 to promote bone repair in skull defects in rabbits. Biomaterials. 2002;23(4):1003–1010. doi: 10.1016/s0142-9612(01)00211-3. [DOI] [PubMed] [Google Scholar]
- 86.Tabata Y., Yamada K., Miyamoto S., et al. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1998;19(7-9):807–815. doi: 10.1016/S0142-9612(98)00233-6. [DOI] [PubMed] [Google Scholar]
- 87.Phillips F. M., Turner A. S., Seim H. B., III, et al. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine Journal. 2006;6(5):500–506. doi: 10.1016/j.spinee.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 88.Sojo K., Sawaki Y., Hattori H., Mizutani H., Ueda M. Immunohistochemical study of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2, -4 (BMP-2, -4) on lengthened rat femurs. Journal of Cranio-Maxillofacial Surgery. 2005;33(4):238–245. doi: 10.1016/j.jcms.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Uchida S., Sakai A., Kudo H., et al. Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone. 2003;32(5):491–501. doi: 10.1016/S8756-3282(03)00053-X. [DOI] [PubMed] [Google Scholar]
- 90.Pufe T., Wildemann B., Petersen W., Mentlein R., Raschke M., Schmidmaier G. Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: a study in the rat. Cell and Tissue Research. 2002;309(3):387–392. doi: 10.1007/s00441-002-0605-0. [DOI] [PubMed] [Google Scholar]
- 91.Cho T.-J., Gerstenfeld L. C., Einhorn T. A. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. Journal of Bone and Mineral Research. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 92.Niikura T., Hak D. J., Hari Reddi A. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. Journal of Orthopaedic Research. 2006;24(7):1463–1471. doi: 10.1002/jor.20182. [DOI] [PubMed] [Google Scholar]
- 93.Blau H. M., Banfi A. The well-tempered vessel. Nature Medicine. 2001;7(5):532–534. doi: 10.1038/87850. [DOI] [PubMed] [Google Scholar]
- 94.Carmeliet P., Jain R. K. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 95.Richardson T. P., Peters M. C., Ennett A. B., Mooney D. J. Polymeric system for dual growth factor delivery. Nature Biotechnology. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 96.Benjamin L. E., Golijanin D., Itin A., Pode D., Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. The Journal of Clinical Investigation. 1999;103(2):159–165. doi: 10.1172/jci5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Samorezov J. E., Headley E. B., Everett C. R., Alsberg E. Sustained presentation of BMP-2 enhances osteogenic differentiation of human adipose-derived stem cells in gelatin hydrogels. Journal of Biomedical Materials Research—Part A. 2016;104(6):1387–1397. doi: 10.1002/jbm.a.35668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park K. Biodegradable thermosensitive polymer gel for sustained BMP-2 delivery. Journal of Controlled Release. 2015;209:p. 337. doi: 10.1016/j.jconrel.2015.05.285. [DOI] [PubMed] [Google Scholar]
- 99.Seo B.-B., Choi H., Koh J.-T., Song S.-C. Sustained BMP-2 delivery and injectable bone regeneration using thermosensitive polymeric nanoparticle hydrogel bearing dual interactions with BMP-2. Journal of Controlled Release. 2015;209:67–76. doi: 10.1016/j.jconrel.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 100.Faßbender M., Minkwitz S., Strobel C., Schmidmaier G., Wildemann B. Stimulation of bone healing by sustained bone morphogenetic protein 2 (BMP-2) delivery. International Journal of Molecular Sciences. 2014;15(5):8539–8552. doi: 10.3390/ijms15058539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poldervaart M. T., Wang H., Van Der Stok J., et al. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0072610.e72610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Springer M. L., Chen A. S., Kraft P. E., Bednarski M., Blau H. M. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Molecular Cell. 1998;2(5):549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- 103.Lee R. J., Springer M. L., Blanco-Bose W. E., Shaw R., Ursell P. C., Blau H. M. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102(8):898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 104.Engsig M. T., Chen Q.-J., Vu T. H., et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. Journal of Cell Biology. 2000;151(4):879–889. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang Q., McHugh K. P., Patntirapong S., Gu X., Wunderlich L., Hauschka P. V. VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and β3-integrin. Matrix Biology. 2008;27(7):589–599. doi: 10.1016/j.matbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Henriksen K., Karsdal M., Delaissé J.-M., Engsig M. T. RANKL and vascular endothelial growth factor (VEGF) induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. The Journal of Biological Chemistry. 2003;278(49):48745–48753. doi: 10.1074/jbc.m309193200. [DOI] [PubMed] [Google Scholar]
- 107.Weltermann A., Wolzt M., Petersmann K., et al. Large amounts of vascular endothelial growth factor at the site of hemostatic plug formation in vivo. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(7):1757–1760. doi: 10.1161/01.ATV.19.7.1757. [DOI] [PubMed] [Google Scholar]
- 108.Pintucci G., Froum S., Pinnell J., Mignatti P., Rafii S., Green D. Trophic effects of platelets on cultured endothelial cells are mediated by platelet-associated fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) Thrombosis and Haemostasis. 2002;88(5):834–842. [PubMed] [Google Scholar]
- 109.Anitua E., Andia I., Ardanza B., Nurden P., Nurden A. T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thrombosis and Haemostasis. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]