Abstract
Physiological changes of pregnancy imposes higher risk of acute respiratory failure (ARF) with even a slight insult and remains an important cause of maternal and fetal morbidity and mortality. Although pregnant women have different respiratory physiology and different causes of ARF, guidelines specific to ventilatory settings, goals of oxygenation and weaning process could not be framed due to lack of large-scale randomized controlled trials. During the 2009 H1N1 pandemic, pregnant women had higher morbidity and mortality compared to nonpregnant women. During this period, alternative strategies of ventilation such as high-frequency oscillatory ventilation, inhalational of nitric oxide, prone positioning, and extra corporeal membrane oxygenation were increasingly used as a desperate measure to rescue pregnant patients with severe hypoxemia who were not improving with conventional mechanical ventilation. This article highlights the causes of ARF and recent advances in invasive, noninvasive and alternative strategies of ventilation used during pregnancy.
Key words: Intensive Care Unit, mechanical ventilation, physiological changes, pregnancy, respiratory failure
Introduction
Critical conditions warranting admission of pregnant patients to Intensive Care Unit (ICU) are relatively rare. The reported incidence varies between 0.4% and 16% of all ICU admissions. Acute respiratory failure (ARF) is the most frequent organ dysfunction associated with ICU admission and has dire maternal and fatal consequences if left untreated.[1,2] Pregnant women have unique characteristics, and their management is challenged by the altered cardio-respiratory physiology, the presence of growing fetus, and diseases which are specific to pregnancy.[3]
Methodology
A thorough and comprehensive literature search in medical databases (PubMed, Google Scholar, and Ovid MEDLINE) was performed with step-wise changes in relevant keywords (pregnancy, physiological changes, ICU, ARF, mechanical ventilation [MV]) without any data restriction including case reports, observational studies, randomized controlled trials (RCTs), and review articles. However, most of the evidence we found in the literature was in the form of case reports, case series, and review articles.
Physiological Changes in Respiratory System
Physiological changes during pregnancy occur due to hormonal effects, mechanical effects of the enlarging uterus, and increased metabolic demands of the fetoplacental unit. These changes, along with a decrease in functional residual capacity (FRC), and impairment in native immunity put parturients at higher risk of ARF with even a slight insult.[4] Respiratory physiology differs in terms of changes in lung volumes, mechanics of ventilation and control of respiration.[4,5]
With the progress of pregnancy, the circumference of the lower chest wall increases by 5–7cm with an increase in the anteroposterior and transverse diameters, resulting in widening of the costal angle from 68° to 103°.[6] This compensates for the eventual elevation of the diaphragm. Lung compliance itself does not change during pregnancy, but chest wall and total respiratory compliances decrease by approximately 30%.[6]
Major changes occurring in lung volumes are summarized in Table 1.[4,5] As respiratory rate (RR) remains unchanged, tachypnea often is a sign of underlying pathology even in parturients. Increase in tidal volume (TV) accounts for mild respiratory alkalosis. The normal partial pressure of carbon dioxide (PaCO2) during pregnancy is 27–34 mmHg. This is due to the stimulatory effects of progesterone on the respiratory center.[7] The partial pressure of oxygen (PaO2) ranges between 90 and 110 mmHg.[4,5] Increase in the oxygen (O2) demand coupled with a decrease in FRC causes smaller airways to close earlier when lung volume is reduced; putting pregnant patients at risk of rapid desaturation.[8]
Table 1.
Changes in lung volumes and capacities during pregnancy

Delivery of O2 to the fetus is affected by maternal delivery of O2 to the placenta, and placental transfer. Frequent and severe episodes of hypoxia lead to low birth weight, intra uterine growth retardation (IUGR), preterm delivery, and increased perinatal morbidity and mortality.[8] The literature recommends maternal PaO2>75 mmHg to protect the fetus from hypoxic injury.[9] Fetal PaCO2 of 65 mmHg or above is associated with detrimental effects. However, persistent respiratory alkalosis (pH >7.48) also causes uterine artery vasoconstriction and decreases fetal perfusion.[10]
Obstetric Causes of Acute Respiratory Failure
In an epidemiological survey, Wanderer et al.[2] found ARF to be the most frequent organ dysfunction (24.6%) present in obstetric patients at the time of ICU admission. The common causes are summarized in Table 2.[9,11,12,13,14,15,16,17]
Table 2.
Causes of acute respiratory failure during pregnancy
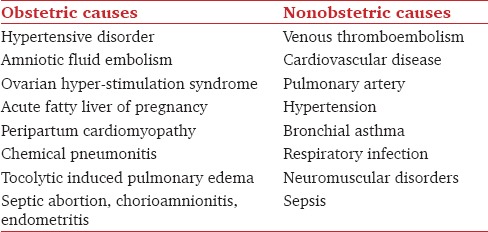
Hypertensive disorders
Hypertensive disorders during pregnancy are classified into four categories: Chronic hypertension, preeclampsia-eclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension.[18] It is the most common medical problem encountered during pregnancy, and also a common indication for ICU admission.[2] Pregnancy-induced hypertension (PIH) complicates 5-14% of all pregnancies and account for a maternal mortality rate (MMR) of 15–20%.[19] Increases in hydrostatic pressure from hypertension and alterations in capillary membrane permeability, leads to pulmonary edema in nearly 3% of women typically after delivery, when plasma oncotic pressure is at its lowest.
Amniotic fluid embolism
Amniotic fluid embolism (AFE) or anaphylactic syndrome of pregnancy is a rare syndrome with high MMR, estimated to occur in 1-12/100,000 deliveries.[20] It is now thought to involve an inflammatory anaphylactoid response to fetal antigens entering the maternal vasculature through disruption in the maternal-fetal barrier. This results in a clinical trial of increased pulmonary and/or systemic vascular resistance decreased left ventricular function and coagulopathy, resulting into cardio-respiratory failure and shock.[21] Predisposing conditions include rapid labor, meconium-stained amniotic fluid, postterm pregnancy, eclampsia, cesarean section (CS), and placental abruption.[22] Management is primarily supportive and is focused on the judicious use of intravenous fluids, hemodynamic monitoring, MV, and administration of blood products for correction of coagulopathy.[21] MMR exceed 60% with classical presentation and increases to 90% if complicated by cardiac arrest. A significant number of survivors had neurologic sequelae from hypoxic-ischemic encephalopathy.[21,23]
Air embolism
Air embolism can occur with normal labor, placenta praevia, criminal abortions, insufflation of the vagina during gynecological procedures, and douching. The mechanism is attributed to entry of air into the sub-placental venous sinuses. A large amount of air collected in the right ventricle can lead to sudden death by blocking the outflow tract. Entry of air into the pulmonary vasculature can cause pulmonary artery hypertension (PAH), right heart failure (RHF) and pulmonary embolism (PE).[24]
Ovarian hyperstimulation syndrome (OHSS)
OHSS is an iatrogenic, life-threatening complication of ovarian stimulation which is being increasingly recognized due to the higher number of women undergoing assisted reproductive techniques. It is associated with the use of gonadotropins, or occasionally clomiphene citrate. The increase in capillary permeability is believed to be the pathognomic sign of this syndrome leading to complications such as ascites, hydrothorax, and anasarca. The clinical features include rapid weight gain, abdominal pain, nausea, vomiting, breathlessness, thromboembolism, hepatic, and renal failure. About 1–2% of women develop a severe form of OHSS warranting ICU admission and aggressive treatment with crystalloids, colloids, albumin, loop diuretics, anticoagulants, cabergoline, and gonadotropin-releasing hormone antagonist. MV is required to manage PE, acute respiratory distress syndrome (ARDS), intra-alveolar hemorrhage and sepsis.[25]
Acute fatty liver of pregnancy
It is a rare clinical entity with an incidence of 1 in 13,000[26] affecting pregnant women in their 3rd trimester. It can lead to hepatic failure, encephalopathy, ARDS, coagulopathy, diabetes insipidus and hypoglycemia. It is histologically and clinically similar to Reye's syndrome, a disease of microvesicular fatty infiltration caused by abnormal oxidation of mitochondrial fatty acids. Liver biopsy is the gold standard diagnostic test, but given the potential for coagulopathy, a biopsy should only be used when the diagnosis is unclear. No specific management is available for this fulminant disorder. However, successful orthotopic liver transplant in a woman with acute fatty liver of pregnancy has been reported.[27]
Peripartum cardiomyopathy
It is a rare form of serious, potentially life-threatening heart disease of uncertain etiology occurring in 3rd trimester of pregnancy or up to 5 months after delivery.[28] The reported incidence is very low (1 in 15,000 deliveries) with an MMR of 20–50%.[29] Recent data have shown that unbalanced peri/post-partum oxidative stress leads to proteolytic cleavage of the hormone prolactin causing pro-apoptotic, and inflammatory changes leading to cardiomyopathy.[28] Restricted sodium diet, loop diuretics, vasodilators, inotropes, beta-blockers, angiotensin converting enzyme inhibitors, immunoglobulins, ventricular assist devices, and heart transplantation have been used for the management.
Chemical pneumonitis
Mendelson's syndrome occurs secondary to aspiration of gastric contents following induction of general anesthesia or in the early postoperative period, resulting in ARDS and PE. Parturients are at high risk of aspiration due to decreased tone of the lower gastro-esophageal sphincter, increased intra-gastric pressure, and decreased gastric emptying. Few hours after aspiration, there can be hypoxia, cyanosis, dyspnea, tachycardia, and shock. There can be bloody, frothy sputum with marked pulmonary congestion. PE may supervene, with a rapidly deteriorating course resulting in death.[30] The risk may be reduced by administering a nonparticulate antacid (e.g., sodium citrate) or an H2-antagonist like ranitidine and by performing rapid sequence induction (RSI). However, the use of high volume of antacid can itself induce vomiting and the effectiveness of RSI has been questioned recently.[31] Increase in the use of regional anesthesia has significantly decreased the incidence of Mendelson's syndrome.
Anemia
Iron deficiency and malnutrition are important causes of anemia in developing countries. Severe anemia of pregnancy is associated with poor outcome. It can lead to palpitations, tachycardia, breathlessness, increased cardiac output leading to increased workload resulting in cardiac de-compensation and failure. Increased incidence of preterm labor (28.2%), preeclampsia (31.2%), and sepsis have also been associated with anemia.[32]
Nonobstetric Causes of Acute Respiratory Failure during Pregnancy
Venous thromboembolism
Pulmonary venous thromboembolism (VTE) complicates 0.05–0.3% of all pregnancies and is the leading cause of maternal death in the developed world.[33,34] Hypercoagulation and venous stasis occur as physiological changes during pregnancy, putting pregnant women at higher risk (relative risk of 4.3) for PE than compared to nonpregnant women.[35] Risk factors include heart disease, age >35 years, CS, sickle cell anemia, diabetes, smoking, multiple pregnancy, obesity, oral contraceptives, hormonal replacement therapy, and personal or family history of VTE.[34]
Echocardiography, ventilation-perfusion scan, spiral computed tomography pulmonary angiography, blood gases, and venous Doppler of lower limbs may help to reach the diagnosis. Therapeutic anticoagulation with unfractionated or low molecular weight heparin (LMWH) should be initiated in the absence of contraindications.[36] Intravenous unfractionated heparin is the initial treatment of choice as it does not cross the placenta and has a short half-life so that it can be titrated before vaginal delivery or CS. LMWH is the agent of choice in the antenatal period. Warfarin is, however, safe in breastfeeding mother and can be commenced between 5 and 7 days postdelivery. Treatment with anticoagulants should ideally be continued for 3–6 months. Although experience with thrombolytic therapy in pregnancy is limited, use of such agents may be lifesaving in patients with hemodynamic compromise.[36,37] Use of temporary vena caval filters, percutaneous mechanical clot fragmentation, compression stockings and cardiopulmonary bypass with surgical embolectomy have also been reported.[36]
Pulmonary artery hypertension
PAH during pregnancy carries high mortality (30–50%) irrespective of its etiology.[38] Severe PAH precipitates RHF resulting in decreased cardiac output and sudden death. Echocardiography is the most useful imaging modality. Epoprostenol, an intravenous prostaglandin is often considered as the first-line therapy.[39] Phosphodiesterase-5 inhibitors such as sildenafil has also been used safely during pregnancy.[40] Endothelin receptor antagonists such as bosentan and sitaxsentan have teratogenic effects, and hence, should be avoided. Even though newer therapies have emerged in the last decade for the management of PAH, significant risk still persists and current guidelines recommend that pregnancy is best avoided or terminated in women with severe PAH of any cause.[41] Hospitalization at 20 weeks of gestation with close monitoring, bed rest, anticoagulants, pulmonary vasodilators, and joint management by obstetricians, cardiologists, and anesthesiologist is recommended for patients who wish to continue their pregnancy.[41]
Bronchial asthma
The prevalence of bronchial asthma varies between 3% and 12% of all pregnancies and approximately 6% of patients get hospitalized due to acute exacerbation.[42] Major causes worsening the severity include nonadherence to medications and viral infections leading to IUGR, preterm labor, preeclampsia, oligohydramnios, and pneumonia.[43] Schatz et al.[44] found that obstetric patients whose bronchial asthma was actively managed had similar maternal and fetal outcomes as compared to those without bronchial asthma. Inhaled bronchodilators and corticosteroids can be safely used during pregnancy as they are less likely to cross the placenta and affect the fetus. Bakhireva et al.[45] examined the effect of inhaled and systemic corticosteroids, and beta-2 agonists on fetal growth. The mean birth weight of infants born to mothers treated with systemic corticosteroids resulted in a deficit of about 200 g compared with controls and exclusive beta-2 agonist users and no increased incidence of small for gestation age. The authors concluded that use of beta-2 agonists and/or inhaled corticosteroids during pregnancy does not impair fetal growth, whereas systemic corticosteroids have a minimal effect which should be weighed against the necessity to control severe asthma. However, theophylline is not usually preferred during pregnancy.
Peak expiratory flow rate (PEFR) does not change with pregnancy and bedside tools (Wright's spirometer and Debono's whistleblowing tests) can be used as simple diagnostic and prognostic indicators.[46] Clinical parameters such as tachycardia (heart rate >120/min), tachypnea (RR >30/min), use of accessory muscles of respiration, excessive sweating, orthopnea, impaired consciousness, pulsus paradoxus, and PEFR <120 L/min indicate the need for hospitalization.[47] Initial management consists of repeated administration of beta-2-agonists, corticosteroids, and supplemental O2. Rising levels of PaCO2(including normalization in a previously hypocapnic patient), exhaustion, impaired consciousness, hemodynamic instability and refractory hypoxemia warrants ICU admission and MV. Use of helium in combination with O2 to facilitate gas exchange and to limit peak inflating pressures has also been reported.[48]
Restrictive lung disease
Women with mild to moderate restrictive lung disease (RLD) tolerate pregnancy reasonably well, but many have a premature delivery. Patients with severe RLD (vital capacity <1 L/min) should be advised to avoid pregnancy or consider a therapeutic abortion. If patient wishes to continue with the pregnancy, optimal medical management of the underlying disease and delivery by CS should be considered. The postoperatively patient may require MV.
Trauma
Trauma has become one of the most frequent causes of maternal death (36%) in the developed world. Road traffic accidents account for 70% of all traumas in pregnant patients.[49] The principle guiding therapy must be that resuscitating the mother will resuscitate the fetus. The commonest obstetric problem caused by trauma is uterine contractions. Placental abruption after trauma occurs in 2–4% of minor accidents and in up to 50% of major injuries.[50] Left lateral tilt at the earliest opportunity should precede primary survey. The patient may require MV for tension pneumothorax, lung contusions, flail chest, and hemodynamic instability.
Respiratory infection
The organisms causing bacterial pneumonia during pregnancy are the same as those found in the nonpregnant state. Pneumococcal pneumonia followed by mycoplasma pneumonia are the usual organisms isolated. In hospitals, nosocomial infection with Gram-negative organism must be considered. Varicella pneumonia is associated with a high mortality of 45% during pregnancy compared with 20% in nonpregnant patients.[51] Among the fungal infections, Coccidioides immitis can affect 1 in 1000 pregnant women.[51] The patient may remain asymptomatic or develop transient pneumonitis. In a retrospective study, Benedetti et al.[52] reported an MMR of 40/1000 deliveries due to antepartum pneumonia.
During the 2009 H1N1 pandemic, pregnant women had higher morbidity and mortality compared to nonpregnant women. There have also been reports of increased risk of miscarriage, birth defect, and preterm delivery.[15,53] The complications were more frequent with advanced gestation.[54] In addition to antivirals and antipyretics, respiratory support may be required with supplemental O2, MV and alternative strategies of ventilation. H1N1 influenza per se is not an indication for delivery; nevertheless, CS was often performed to improve maternal oxygenation and respiratory function rather salvage a compromised fetus.
Acute respiratory distress syndrome
ARDS can result from or modified by an obstetric factor [Table 3].[9] The reported incidence varies between 1 per 6000 and 10,000 deliveries, occurring primarily in the 3rd trimester.[9,55,56] Perry et al.[57] found an MMR of 30-50% and perinatal mortality of 20–25%. Arterial blood gas (ABG) criteria for intubation may vary depending on the gestational age. Inability to maintain a PaO2 of 70 mmHg or SpO2 of 95% with conservative therapy suggests respiratory compromise and warrants intensive therapy.[58] High rates of fetal death, spontaneous preterm labor, and fetal heart rate (FHR) abnormalities are reported in neonates born to these pregnant women.[51]
Table 3.
Causes of acute respiratory distress syndrome during pregnancy
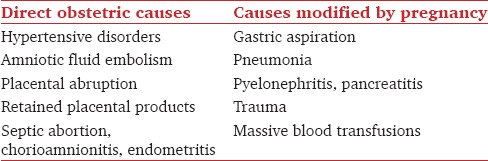
Pulmonary edema
Acute PE in pregnant women is an uncommon but life-threatening event. Incidence varies from 0.08% to 3%[59,60,61] [Table 4].
Table 4.
Causes of pulmonary edema

Iatrogenic fluid overload is the most common cause of PE as pregnant women already have increased blood volume and are vulnerable to volume overload. Sodium and water retention secondary to oxytocin administered during delivery and preexisting cardiac abnormalities such as valvular heart disease, congenital heart disease including Eisenmenger syndrome, coarctation of the aorta, and cardiomyopathy further precipitates the condition.
Tocolytics are used to delay preterm labor if it occurs between 24 and 34 weeks of gestation and use of glucocorticoids is likely to enhance the lung maturity, provided there is no contraindication to their use, and where a delay in delivery of the newborn is likely to improve neonatal outcome. The incidence of tocolytic therapy induced pulmonary edema is approximately 1 in 400 patients receiving beta-adrenergic agents.[13] It can occur during the treatment or up to 12 h after the discontinuation of the drug. These adrenergic drugs cause vasodilatation and tachycardia resulting in hypotension. Treatment with intravenous fluids predisposes already fluid overloaded patient into PE. By acting on the beta-receptors in the proximal tubules of the kidney, these drugs stimulate renin and antidiuretic hormone synthesis resulting in sodium and fluid retention. Patients on tocolytic treatment also receive steroids to accelerate fetal lung maturation, and their mineralocorticoid activity aggravates the condition.[13] Treatment consists of discontinuation of tocolytic drug therapy, administration of loop diuretics and supplemental O2. Use of both noninvasive and invasive MV has also been reported.[62]
Mechanical Ventilation
During the initial stages of maternal decompensation, the best support for the fetus is to maintain a nonhostile intrauterine environment. The physiological changes of pregnancy like an increase in O2 demand, respiratory alkalosis, a decrease in FRC and decrease in respiratory compliance should be taken into account. The presence of mucosal edema, capillary engorgement, and increase in breast size, warrant a 0.5 mm smaller endotracheal tube compared to nonpregnant women of similar height and age. Start with a 7 mm endotracheal tube for the average-size pregnant woman. Although a PaO2 of 55 mmHg and SpO2 of 88% would be tolerated in the general population, adequate fetal oxygenation requires a PaO2 of 70 mmHg, which corresponds to a maternal SpO2 of about 95%.[9] Clearance of fetal PaCO2 by the placenta requires a gradient of approximately 10 mmHg.[10] Limited data suggest that PaCO2 levels of 45–55 mmHg is reasonable in the later part of pregnancy.
Noninvasive ventilation
Noninvasive ventilation (NIV) has been successfully used during pregnancy to treat ARF resulting from bronchial asthma, sickle cell anemia, all-trans-retinoic-acid syndrome, tocolytic induced pulmonary edema, influenza, community acquired pneumonia, ARDS, etc.[10,63,64,65,66] It avoids the potential complications of endotracheal intubation, and that of the drugs used for sedation. Usually, an inspiratory pressure of 12–15 cmH2O and an expiratory pressure of 5–8 cmH2O are used.[66] Its implementation under close monitoring would shorten hospitalization and ICU stay. However, decreased tone of the lower gastroesophageal sphincter, increased intragastric pressure and decreased gastric emptying put parturients at higher risk of gastric aspiration. Positive pressure ventilation through face mask may lead to gastric distension and vomiting.[3] Therefore, NIV should be reserved for patients who are alert and conscious, have protective airway reflexes, good respiratory drive, and stable hemodynamics with no severe acid-base disturbances.
Invasive ventilation
Specific guidelines on ventilatory settings, goals of oxygenation and weaning process are still lacking during pregnancy.[67] Initiation of MV in a pregnant patient with ARDS should follow the guidelines of the ARDS network study.[68]
If respiratory acidosis (PaCO2>65 mmHg) persists despite high RR, the TV can be increased as long as plateau pressure remains <30 cmH2O.[69] Though it could be argued that decrease in the chest wall compliance should allow for an increase in the plateau pressures limit, Campbell et al.[69] were of the opinion that ARDS network guidelines should not be altered, as it is the lung compliance which becomes the major determinant of total respiratory compliance rather than the effect of pregnancy on chest wall compliance.
During the influenza pandemic of 2009, Pollock et al.[70] compared the provision of MV to pregnant/postpartum women with a nonpregnant matched control group. They studied 36 cases and 38 controls. There were no differences in the preintubation and postintubation ABG values apart from a lower PaCO2 and bicarbonate levels in cases. Initial ventilator settings including mode, TV, and RR demonstrated no differences between the cases and controls. MMR did not significantly differ between the two groups (52.6% vs 48.3%).
Alternative strategies of ventilation
Extra corporeal membrane oxygenation (ECMO)
Early initiation of ECMO is recommended if ARF with refractory hypoxia is encountered in a pregnant woman. It saves the life of the mother but exposes the fetus to complications of systemic heparinization and extracorporeal circulation. The decision to perform CS while the patient is on ECMO is not easy due to the increased risk of thrombosis from the required duration of heparin discontinuation and risk of bleeding if heparin infusion is continued. Nair et al.[71] retrospectively studied 12 critically ill pregnant and postpartum women suffering from severe ARDS during the H1N1 pandemic who required ECMO therapy. The technical challenges, efficacy, complications and maternal/fetal outcomes were evaluated. ECMO circuit related complications were rare, circuit change was needed in only two cases, and there was no sudden circuit failure. However, bleeding was common, leading to large volumes of blood cell transfusion and was the main cause of mortality.
Prone position
In addition to usual care, pregnant women require greater emphasis on avoiding any external abdominal pressure, continuous fetal monitoring and highly dedicated supporting staff. In addition prone positioning is logistically challenging even in nonpregnant patients and benefits are short and do not last once the position is changed to supine. However, case reports of successful use of prone ventilation in pregnancy with ARDS are reported.[72]
High-frequency oscillatory ventilation
It is a lung protective form of ventilation which was used as a rescue therapy in up to 12% of patients with H1N1 associated ARDS who initially showed no signs of improvement with antivirals and conventional MV.[73]
Nitric oxide
It is not known whether inhalational of nitric oxide (iNO) can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity.[74] It is also not known whether NO is excreted in human milk or not and is not approved for use by Food and Drug Administration (FDA). However, Michael et al.[75] reported a case of successful use of iNO of up to 40 parts per million for 2 days in a pregnant patient with severe ARDS.
There are few case reports supporting the use of alternative strategies of ventilation like high-frequency oscillatory ventilation, iNO, prone positioning, and ECMO during the 2009 pandemic of H1N1 influenza as a desperate measure to rescue pregnant patients with severe hypoxemia who were not improving with conventional MV.
Weaning
Weaning parameters for pregnant patients are not well-established, but it seems reasonable to follow the same guidelines as for nonpregnant patients.[76]
Sedation and Paralysis
RCTs evaluating drugs for providing long-term sedation, analgesia, and neuromuscular blockade are lacking in pregnancy. Benzodiazepines freely cross the placenta and may accumulate in the fetus. Use of diazepam in early pregnancy has been linked with a small risk of cleft lip and palate.[77] Midazolam and lorazepam appear to cross the placenta to a lesser degree than diazepam, although the clinical significance is unknown.[78] There are no large data on the use of dexmedetomidine during pregnancy. Few case reports described its use for sedation near term and all patient's required CS.[79] Higher doses (0.5 μg/kg/h) or prolonged duration of infusion lead to fetal bradycardia. Propofol has been assigned to pregnancy category-B by the FDA. It rapidly crosses the placenta and distributed into fetal circulation. Higher doses (2.8 mg/kg) can result in low Apgar scores, muscle hypotony, and depressed neuromuscular activity. But by large no difference was found in the Apgar scores of infants exposed to propofol during CS. Animal studies failed to reveal fetal harm or impaired fertility. There are no RCTs conducted in human pregnancy and no data on the prolonged use of propofol is available during pregnancy. Congenital malformations have not been reported with the use of opioids such as morphine and fentanyl. Nondepolarizing neuromuscular blocking agents freely cross the placenta, but found to have no clinical effect on the fetus in the short term.[78]
Fetal Monitoring
During prolonged MV, it is reasonable to measure and record daily FHR tracing, weekly umbilical artery Doppler and every fortnight ultrasound scan for fetal growth and monitoring. When gestation has progressed enough for delivery by CS, amniocentesis may be helpful to determine fetal maturity. If early delivery is not an option, the best course is to focus on optimizing the health of the mother and not on minute-to-minute variations in FHR.
Radiological investigations like chest X-ray are routinely required for the assessment and management of ventilated pregnant patients. A single chest X-ray results in 60 millirads of exposure. Although there is a potential risk of exposing the fetus to radiation, shielding the abdomen with lead and using a well-collimated X-ray beam can reduce fetal exposure.[80] Radiation exposure >0.05–0.5 Gy (5-50 rads) leads to teratogenicity and fetal demise in 1st trimester, gross congenital malformation and mental retardation in 2nd trimester and depletion of cell population in 3rd trimester.[81]
Ethical and Social Issues
Prolonged MV during pregnancy entails significant ethical and management considerations. Even after brain death, few mothers can carry on their pregnancy until term. Hence, end-of-life care decisions are difficult to make with a viable fetus and may understandably be postponed until delivery. Tomlinson et al.[82] noted that early delivery of the fetus reduces maternal O2 requirement by an average of 28% within 24 h. Case reports of prolonged support exceeding 100 days with the successful delivery of a viable fetus are reported in the literature.[83] Preservation of utero-placental blood flow due to the absence of autoregulation of the uterine vasculature is the priority. In the systematic review done by Esmaeilzadeh et al.[84] organ donation from the brain-dead mother was carried out in ten patients with an excellent 1 year graft survival. Conducting RCTs on the mother during pregnancy also involve social and ethical issues and hence are difficult to undertake.
Conclusion
Though pregnant patients have different respiratory physiology and different causes of respiratory failure, guidelines specific to MV in pregnant patients could not be framed and in general the principles of MV that are used for nonobstetric patients are also followed in pregnant patients. Selection of drugs should take into account of its possible teratogenic effects and list of safe drugs should be available in obstetric ICU. Fetal monitoring is an essential part of ICU care and obstetric consultation should be sought on the safety of continuation of pregnancy and method of delivery at the earliest.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pollock W, Rose L, Dennis CL. Pregnant and postpartum admissions to the intensive care unit: A systematic review. Intensive Care Med. 2010;36:1465–74. doi: 10.1007/s00134-010-1951-0. [DOI] [PubMed] [Google Scholar]
- 2.Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, Bateman BT. Epidemiology of obstetric-related ICU admissions in Maryland: 1999-2008. Crit Care Med. 2013;41:1844–52. doi: 10.1097/CCM.0b013e31828a3e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trikha A, Singh P. The critically ill obstetric patient – Recent concepts. Indian J Anaesth. 2010;54:421–7. doi: 10.4103/0019-5049.71041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegewald MJ, Crapo RO. Respiratory physiology in pregnancy. Clin Chest Med. 2011;32:1–13, vii. doi: 10.1016/j.ccm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Dildy GA, Belfort MA, Saade GR, Phelan JP, Hankins GD, Clark SL. Critical Care Obstetrics. 4th ed. New York: Wiley-Blackwell; 2004. Pregnancy-induced physiologic alterations; pp. 19–42. [Google Scholar]
- 6.Bobrowski RA. Pulmonary physiology in pregnancy. Clin Obstet Gynecol. 2010;53:285–300. doi: 10.1097/GRF.0b013e3181e04776. [DOI] [PubMed] [Google Scholar]
- 7.Crapo RO. Normal cardiopulmonary physiology during pregnancy. Clin Obstet Gynecol. 1996;39:3–16. doi: 10.1097/00003081-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Templeton A, Kelman GR. Maternal blood-gases, (Pao2-Pao2), hysiological shunt and VD/VT in normal pregnancy. Br J Anaesth. 1976;48:1001–4. doi: 10.1093/bja/48.10.1001. [DOI] [PubMed] [Google Scholar]
- 9.Catanzarite V, Willms D, Wong D, Landers C, Cousins L, Schrimmer D. Acute respiratory distress syndrome in pregnancy and the puerperium: Causes, courses, and outcomes. Obstet Gynecol. 2001;97(5 Pt 1):760–4. doi: 10.1016/s0029-7844(00)01231-x. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ansari MA, Hameed AA, Al-jawder SE, Saeed HM. Use of noninvasive positive pressure ventilation during pregnancy: Case series. Ann Thorac Med. 2007;2:23–5. doi: 10.4103/1817-1737.30358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mighty HE. Acute respiratory failure in pregnancy. Clin Obstet Gynecol. 2010;53:360–8. doi: 10.1097/GRF.0b013e3181deb3f1. [DOI] [PubMed] [Google Scholar]
- 12.Sciscione AC, Ivester T, Largoza M, Manley J, Shlossman P, Colmorgen GH. Acute pulmonary edema in pregnancy. Obstet Gynecol. 2003;101:511–5. doi: 10.1016/s0029-7844(02)02733-3. [DOI] [PubMed] [Google Scholar]
- 13.Lamont RF. The pathophysiology of pulmonary oedema with the use of beta-agonists. BJOG. 2000;107:439–44. doi: 10.1111/j.1471-0528.2000.tb13259.x. [DOI] [PubMed] [Google Scholar]
- 14.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659–70. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–26. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 16.Martinelli I. Risk factors in venous thromboembolism. Thromb Haemost. 2001;86:395–403. [PubMed] [Google Scholar]
- 17.Ramsey PS, Ramin KD. Pneumonia in pregnancy. Obstet Gynecol Clin North Am. 2001;28:553–69. doi: 10.1016/s0889-8545(05)70217-5. [DOI] [PubMed] [Google Scholar]
- 18.Zamorski MA, Green LA. NHBPEP report on high blood pressure in pregnancy: A summary for family physicians. Am Fam Physician. 2001;64:263–70. [PubMed] [Google Scholar]
- 19.Henry CS, Biedermann SA, Campbell MF, Guntupalli JS. Spectrum of hypertensive emergencies in pregnancy. Crit Care Clin. 2004;20:697–712, ix. doi: 10.1016/j.ccc.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid embolisms: A population-based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199:49.e1–8. doi: 10.1016/j.ajog.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 21.Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337–48. doi: 10.1097/AOG.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 22.Kramer MS, Rouleau J, Liu S, Bartholomew S, Joseph KS. Maternal Health Study Group of the Canadian Perinatal Surveillance System. Amniotic fluid embolism: Incidence, risk factors, and impact on perinatal outcome. BJOG. 2012;119:874–9. doi: 10.1111/j.1471-0528.2012.03323.x. [DOI] [PubMed] [Google Scholar]
- 23.Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: Analysis of the national registry. Am J Obstet Gynecol. 1995;172(4 Pt 1):1158–67. doi: 10.1016/0002-9378(95)91474-9. [DOI] [PubMed] [Google Scholar]
- 24.Lowenwirt IP, Chi DS, Handwerker SM. Nonfatal venous air embolism during cesarean section: A case report and review of the literature. Obstet Gynecol Surv. 1994;49:72–6. doi: 10.1097/00006254-199401000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Arikan I, Barut A, Harma M, Harma MI. A severe case of ovarian hyperstimulation syndrome with pulmonary thromboembolism. Int J Gynecol Obstet. 2009;13:1. [Google Scholar]
- 26.Watson WJ, Seeds JW. Acute fatty liver of pregnancy. Obstet Gynecol Surv. 1990;45:585–91. doi: 10.1097/00006254-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Remiszewski P, Pawlak J, Skwarek A, Grzelak I, Patkowski W, Grodzicki M, et al. Orthotopic liver transplantation for acute liver failure resulting from “acute fatty liver of pregnancy”. Ann Transplant. 2003;8:8–11. [PubMed] [Google Scholar]
- 28.Hilfiker-Kleiner D, Sliwa K, Drexler H. Peripartum cardiomyopathy: Recent insights in its pathophysiology. Trends Cardiovasc Med. 2008;18:173–9. doi: 10.1016/j.tcm.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Tidswell M. Peripartum cardiomyopathy. Crit Care Clin. 2004;20:777–88, xi. doi: 10.1016/j.ccc.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Bandi VD, Munnur U, Matthay MA. Acute lung injury and acute respiratory distress syndrome in pregnancy. Crit Care Clin. 2004;20:577–607. doi: 10.1016/j.ccc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Brimacombe JR, Berry AM. Cricoid pressure. Can J Anaesth. 1997;44:414–25. doi: 10.1007/BF03014464. [DOI] [PubMed] [Google Scholar]
- 32.UNICEF and Micronutrient Initiative. Vitamin and Mineral Deficiency: A Global Progress Report. 2004 Mar [Google Scholar]
- 33.Snow V, Qaseem A, Barry P, Hornbake ER, Rodnick JE, Tobolic T, et al. Management of venous thromboembolism: A clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007;146:204–10. doi: 10.7326/0003-4819-146-3-200702060-00149. [DOI] [PubMed] [Google Scholar]
- 34.Larsen TB, Sørensen HT, Gislum M, Johnsen SP. Maternal smoking, obesity, and risk of venous thromboembolism during pregnancy and the puerperium: A population-based nested case-control study. Thromb Res. 2007;120:505–9. doi: 10.1016/j.thromres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ., 3rd Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann Intern Med. 2005;143:697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- 36.Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:844–86. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- 37.Leonhardt G, Gaul C, Nietsch HH, Buerke M, Schleussner E. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis. 2006;21:271–6. doi: 10.1007/s11239-006-5709-z. [DOI] [PubMed] [Google Scholar]
- 38.Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: A systematic overview from 1978 through 1996. J Am Coll Cardiol. 1998;31:1650–7. doi: 10.1016/s0735-1097(98)00162-4. [DOI] [PubMed] [Google Scholar]
- 39.Bendayan D, Hod M, Oron G, Sagie A, Eidelman L, Shitrit D, et al. Pregnancy outcome in patients with pulmonary arterial hypertension receiving prostacyclin therapy. Obstet Gynecol. 2005;106(5 Pt 2):1206–10. doi: 10.1097/01.AOG.0000164074.64137.f1. [DOI] [PubMed] [Google Scholar]
- 40.Goland S, Tsai F, Habib M, Janmohamed M, Goodwin TM, Elkayam U. Favorable outcome of pregnancy with an elective use of epoprostenol and sildenafil in women with severe pulmonary hypertension. Cardiology. 2010;115:205–8. doi: 10.1159/000287638. [DOI] [PubMed] [Google Scholar]
- 41.Higton AM, Whale C, Musk M, Gabbay E. Pulmonary hypertension in pregnancy: Two cases and review of the literature. Intern Med J. 2009;39:766–70. doi: 10.1111/j.1445-5994.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 42.Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol. 2005;106(5 Pt 1):1046–54. doi: 10.1097/01.AOG.0000185281.21716.02. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Wen SW, Demissie K, Marcoux S, Kramer MS. Maternal asthma and pregnancy outcomes: A retrospective cohort study. Am J Obstet Gynecol. 2001;184:90–6. doi: 10.1067/mob.2001.108073. [DOI] [PubMed] [Google Scholar]
- 44.Schatz M, Zeiger RS, Hoffman CP, Harden K, Forsythe A, Chilingar L, et al. Perinatal outcomes in the pregnancies of asthmatic women: A prospective controlled analysis. Am J Respir Crit Care Med. 1995;151:1170–4. doi: 10.1164/ajrccm/151.4.1170. [DOI] [PubMed] [Google Scholar]
- 45.Bakhireva LN, Jones KL, Schatz M, Johnson D, Chambers CD. Organization of Teratology Information Services Research Group. Asthma medication use in pregnancy and fetal growth. J Allergy Clin Immunol. 2005;116:503–9. doi: 10.1016/j.jaci.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 46.Brancazio LR, Laifer SA, Schwartz T. Peak expiratory flow rate in normal pregnancy. Obstet Gynecol. 1997;89:383–6. doi: 10.1016/S0029-7844(96)00512-1. [DOI] [PubMed] [Google Scholar]
- 47.Cydulka RK, Emerman CL, Schreiber D, Molander KH, Woodruff PG, Camargo CA., Jr Acute asthma among pregnant women presenting to the emergency department. Am J Respir Crit Care Med. 1999;160:887–92. doi: 10.1164/ajrccm.160.3.9812138. [DOI] [PubMed] [Google Scholar]
- 48.George R, Berkenbosch JW, Fraser RF, 2nd, Tobias JD. Mechanical ventilation during pregnancy using a helium-oxygen mixture in a patient with respiratory failure due to status asthmaticus. J Perinatol. 2001;21:395–8. doi: 10.1038/sj.jp.7210530. [DOI] [PubMed] [Google Scholar]
- 49.Neufeld JD. Trauma in pregnancy, what if...? Emerg Med Clin North Am. 1993;11:207–24. [PubMed] [Google Scholar]
- 50.Kuhlmann RS, Cruikshank DP. Maternal trauma during pregnancy. Clin Obstet Gynecol. 1994;37:274–93. doi: 10.1097/00003081-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Flick MR. Textbook of Respiratory Medicine. 2nd ed. Philadelphia: WB Saunders; 1994. The lungs and gynaecologic and obstetric disease; pp. 2475–88. [Google Scholar]
- 52.Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet Gynecol. 1982;144:413–7. doi: 10.1016/0002-9378(82)90246-0. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14:95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC). Hospitalized patients with novel influenza A (H1N1) virus infection – California, April-May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–41. [PubMed] [Google Scholar]
- 55.Mabie WC, Barton JR, Sibai BM. Adult respiratory distress syndrome in pregnancy. Am J Obstet Gynecol. 1992;167(4 Pt 1):950–7. doi: 10.1016/s0002-9378(12)80018-4. [DOI] [PubMed] [Google Scholar]
- 56.Smith JL, Thomas F, Orme JF, Jr, Clemmer TP. Adult respiratory distress syndrome during pregnancy and immediately postpartum. West J Med. 1990;153:508–10. [PMC free article] [PubMed] [Google Scholar]
- 57.Perry KG, Jr, Martin RW, Blake PG, Roberts WE, Martin JN., Jr Maternal mortality associated with adult respiratory distress syndrome. South Med J. 1998;91:441–4. doi: 10.1097/00007611-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Deblieux PM, Summer WR. Acute respiratory failure in pregnancy. Clin Obstet Gynecol. 1996;39:143–52. doi: 10.1097/00003081-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Dunne C, Meriano A. Acute postpartum pulmonary edema in a 23-year-old woman 5 days after cesarean delivery. CJEM. 2009;11:178–81. doi: 10.1017/s1481803500011179. [DOI] [PubMed] [Google Scholar]
- 60.Vigil-De Gracia P, Montufar-Rueda C, Smith A. Pregnancy and severe chronic hypertension: Maternal outcome. Hypertens Pregnancy. 2004;23:285–93. doi: 10.1081/PRG-200030315. [DOI] [PubMed] [Google Scholar]
- 61.Dennis AT, Solnordal CB. Acute pulmonary oedema in pregnant women. Anaesthesia. 2012;67:646–59. doi: 10.1111/j.1365-2044.2012.07055.x. [DOI] [PubMed] [Google Scholar]
- 62.DiFederico EM, Burlingame JM, Kilpatrick SJ, Harrison M, Matthay MA. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or of nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925–33. doi: 10.1016/s0002-9378(98)70190-5. [DOI] [PubMed] [Google Scholar]
- 63.Draisci G, Volpe C, Pitoni S, Zanfini BA, Gonnella GL, Catarci S, et al. Non-invasive ventilation for acute respiratory failure in preterm pregnancy. Int J Obstet Anesth. 2013;22:169–71. doi: 10.1016/j.ijoa.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Bassani MA, de Oliveira AB, Oliveira Neto AF. Noninvasive ventilation in a pregnant patient with respiratory failure from all-trans-retinoic-acid (ATRA) syndrome. Respir Care. 2009;54:969–72. doi: 10.4187/002013209793800466. [DOI] [PubMed] [Google Scholar]
- 65.Mohapatra PR, Dutt N, Khanduri S, Mishra B, Janmeja AK. Noninvasive ventilation in acute respiratory failure due to H1N1 influenza. Lung India. 2011;28:49–51. doi: 10.4103/0970-2113.76301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banga A, Khilnani GC. Use of non-invasive ventilation in a pregnant woman with acute respiratory distress syndrome due to pneumonia. Indian J Chest Dis Allied Sci. 2009;51:115–7. [PubMed] [Google Scholar]
- 67.Jenkins TM, Troiano NH, Graves CR, Baird SM, Boehm FH. Mechanical ventilation in an obstetric population: Characteristics and delivery rates. Am J Obstet Gynecol. 2003;188:549–52. doi: 10.1067/mob.2003.68. [DOI] [PubMed] [Google Scholar]
- 68.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 69.Campbell LA, Klocke RA. Implications for the pregnant patient. Am J Respir Crit Care Med. 2001;163:1051–4. doi: 10.1164/ajrccm.163.5.16353. [DOI] [PubMed] [Google Scholar]
- 70.Pollock WE, Bellomo R, Webb S, Seppelt I, Davies A, Howe B, et al. Provision of mechanical ventilation to pregnant/postpartum women with H1N1 influenza: A case-control study. Aust Crit Care. 2013;26:83. [Google Scholar]
- 71.Nair P, Davies AR, Beca J, Bellomo R, Ellwood D, Forrest P, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med. 2011;37:648–54. doi: 10.1007/s00134-011-2138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samanta S, Samanta S, Wig J, Baronia AK. How safe is the prone position in acute respiratory distress syndrome at late pregnancy? Am J Emerg Med. 2014;32:687.e1–3. doi: 10.1016/j.ajem.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 73.Netteburg D, Bsat F, Healy A, Markenson G, Plevyak M, Circeo L. The use of high-frequency oscillatory ventilation in a patient with H1N1 pneumonia. J Matern Fetal Neonatal Med. 2011;24:264–6. doi: 10.3109/14767058.2010.484470. [DOI] [PubMed] [Google Scholar]
- 74.Robinson JN, Banerjee R, Landzberg MJ, Thiet MP. Inhaled nitric oxide therapy in pregnancy complicated by pulmonary hypertension. Am J Obstet Gynecol. 1999;180:1045–6. doi: 10.1016/s0002-9378(99)70686-1. [DOI] [PubMed] [Google Scholar]
- 75.Michael C, Jennifer WM. Acute Respiratory Distress Syndrome Due to H1N1 Influenza can be Safely Treated with Inhaled Nitric Oxide in a Pregnant Patient. American Thoracic Society International Conference Abstract D52. What's New in Swine Flu? 2010 [Google Scholar]
- 76.Alía I, Esteban A. Weaning from mechanical ventilation. Crit Care. 2000;4:72–80. doi: 10.1186/cc660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: Meta-analysis of cohort and case-control studies. BMJ. 1998;317:839–43. doi: 10.1136/bmj.317.7162.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shehata HA, Nelson-Piercy C. Drugs in pregnancy. Drugs to avoid. Best Pract Res Clin Obstet Gynaecol. 2001;15:971–86. doi: 10.1053/beog.2001.0241. [DOI] [PubMed] [Google Scholar]
- 79.Abu-Halaweh SA, Al Oweidi AK, Abu-Malooh H, Zabalawi M, Alkazaleh F, Abu-Ali H, et al. Intravenous dexmedetomidine infusion for labour analgesia in patient with preeclampsia. Eur J Anaesthesiol. 2009;26:86–7. doi: 10.1097/EJA.0b000e000000f3fb. [DOI] [PubMed] [Google Scholar]
- 80.Diethelm L, Xu H. Diagnostic imaging of the lung during pregnancy. Clin Obstet Gynecol. 1996;39:36–55. doi: 10.1097/00003081-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Bentur Y, Horlatsch N, Koren G. Exposure to ionizing radiation during pregnancy: Perception of teratogenic risk and outcome. Teratology. 1991;43:109–12. doi: 10.1002/tera.1420430203. [DOI] [PubMed] [Google Scholar]
- 82.Tomlinson MW, Caruthers TJ, Whitty JE, Gonik B. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol. 1998;91:108–11. doi: 10.1016/s0029-7844(97)00585-1. [DOI] [PubMed] [Google Scholar]
- 83.Feldman DM, Borgida AF, Rodis JF, Campbell WA. Irreversible maternal brain injury during pregnancy: A case report and review of the literature. Obstet Gynecol Surv. 2000;55:708–14. doi: 10.1097/00006254-200011000-00023. [DOI] [PubMed] [Google Scholar]
- 84.Esmaeilzadeh M, Dictus C, Kayvanpour E, Sedaghat-Hamedani F, Eichbaum M, Hofer S, et al. One life ends, another begins: Management of a brain-dead pregnant mother-A systematic review. BMC Med. 2010;8:74. doi: 10.1186/1741-7015-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]


