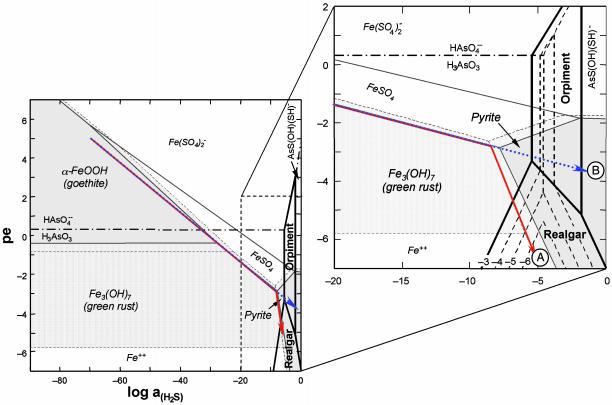
Fig. 4.
Reaction path model. Quantitative model for the schematic reactions shown in Fig. 3 illustrated as pe-log aH2S stability diagram for the As-Fe-S-O-H system at 25°C and pH = 7. Log activity (S)total = –2; iron mineral stability fields are shown for log activity (Fe)total = –5 (stability fields shaded gray) and –7 (stability fields stippled); and arsenic mineral stability domains are calculated for log activity (As)total = –3 to –6, as labeled. Reaction paths are shown for evolution of iron-rich (path A, solid red arrow) and iron-poor (path B, dotted blue arrow) sediments during oxidation of organic matter. Equilibrium reaction paths were calculated for evolution of groundwater containing 28 mmol·kg–1  and 100 μmol·kg–1 as during progressive oxidation of sedimentary organic matter in iron-rich (100 mmol·kg–1 reactive Fe) and iron-poor (1 mmol·kg–1 reactive Fe) sediment. At log activity (Fe)total =–7, the stability field for green rust is absent and iron exists as Fe2+(aq).
and 100 μmol·kg–1 as during progressive oxidation of sedimentary organic matter in iron-rich (100 mmol·kg–1 reactive Fe) and iron-poor (1 mmol·kg–1 reactive Fe) sediment. At log activity (Fe)total =–7, the stability field for green rust is absent and iron exists as Fe2+(aq).