Abstract
Transporter genes and cytokinins are key targets for crop improvement. These genes are active during the development of the seed and its establishment as a strong sink. However, during germination, the seed transitions to being a source for the developing root and shoot. To determine if the sucrose transporter (SUT), amino acid permease (AAP), Sugar Will Eventually be Exported Transporter (SWEET), cell wall invertase (CWINV), cytokinin biosynthesis (IPT), activation (LOG) and degradation (CKX) gene family members are involved in both the sink and source activities of seeds, we used RT-qPCR to determine the expression of multiple gene family members, and LC-MS/MS to ascertain endogenous cytokinin levels in germinating Pisum sativum L. We show that genes that are actively expressed when the seed is a strong sink during its development, are also expressed when the seed is in the reverse role of being an active source during germination and early seedling growth. Cytokinins were detected in the imbibing seeds and were actively biosynthesised during germination. We conclude that, when the above gene family members are targeted for seed yield improvement, a downstream effect on subsequent seed germination or seedling vigour must be taken into consideration.
Keywords: cytokinin, germination, Pisum sativum
1. Introduction
The dynamic relationship between sources and sinks changes markedly during the life cycle of the plant. Leaves commence their life cycle initially as sinks, and mature into sources, and the seed, initially a strong sink, becomes the source of energy and nutrients during germination [1]. The availability and partitioning of carbon and nitrogen (N) provide the resources underpinning source-sink dynamics [2,3].
Key to the movement of sucrose around the plant are the Sugar Will Eventually be Exported Transporters (SWEETs), sucrose transporters (SUTs) and cell wall invertases (CWINVs) [1,4,5], and to the movement of amino acids are the amino acid permeases (AAPs) [6,7]. These same proteins are involved in the transport of assimilates to the developing seed [8,9,10,11,12]. However, during germination, as the seed changes from a sink to a source, the question arises as to whether these proteins are involved in the re-mobilisation of the reserves they helped to mobilise to the seed in the first place.
Functional studies have shown that the Arabidopsis AAPs can transport a wide range of amino acids [6,13], and various gene family members are involved in the source to sink translocation of amino acids [7]. In pea, expression of the AtAAP1::PsAAP1 construct increased overall plant biomass and N content, as well as increasing seed yield and seed N [11]. Seed storage proteins are degraded to provide amino acids for the biosynthesis of nucleic acids and new proteins, but may also be metabolised to satisfy the energy demands of the developing seedling [14,15]. Transportation of amino acids within the germinating seed and to the young seedling would appear to be a necessary requirement. In both the legume, Medicago truncatula, and in rice, the AAPs constitute larger gene families than in Arabidopsis [13,16]. We showed previously that of the 13 PsAAP gene family members detected in a pea transcriptome, the PsAAPs were represented in most of the clusters of AAP orthologues in Arabidopsis and other leguminous species [17]. Multiple sequences were identified in several family members, particularly for PsAAP2 (Cluster 3A) and PsAAP7 (Cluster 1), as previously noted [13]. AtAAP7 has yet to be functionally characterised.
In contrast to the AAPs, SUTs belong to a smaller gene family [18]. SUTs are considered essential to the movement of sucrose from source leaves to sink organs, and to the uploading of sucrose into seeds [9,19,20]. Type I and II SUTs are localised to the plasma membrane [20]. The role of SUTs during early seed germination has been studied in rice [21,22]. In monocots, Type II SUTs (OsSUT1, 3, and 4) are utilised for phloem loading [20,23]. During early seed germination, OsSUT1 is upregulated in the scutellar vascular bundle [21], while OsSUT4 is expressed in the seed aleurone layer, and subsequently in the scutellum and embryonic vascular bundle [22], indicating differential temporal and spatial expression for these gene family members during germination.
In Ricinus communis, sucrose is released from the endosperm to the apoplast and from there taken up by the cotyledons for transfer to the rest of the seedling [24]. RcSUT1 was shown to be expressed more in the cotyledon of the germinating seed than in the endosperm [24]. While the loading of sucrose into developing legume seeds has been studied in detail [8,9,19,25,26], it would appear less attention has been given to the movement of sugars during the germination of non-endospermous seed, such as pea.
SWEETs are the most recent gene family to be designated a key role in sink/source dynamics [27,28]. Dhandapani et al. (2016) [17] showed that the 13 PsSWEET gene sequences identified from a pea transcriptome had members in all four of the SWEET clades described recently [12,29]. SWEETs have been strongly linked to development in reproductive tissues, especially seeds [12,30,31]. Transgenic analysis has indicated that SWEETs may be activated during seed germination. Seeds over-expressing AtSWEET16 (a Clade IV SWEET) germinated faster than controls [31] as did those over-expressing AtSWEET4 (a Clade II SWEET) [32]. However, the expression of endogenous SWEETs during germination has yet to be shown.
Cell wall invertases catalyse the irreversible breakdown of sucrose to fructose and glucose, and are an integral component of the movement of sucrose between sources and sinks [1,33,34]. They have been shown to be up-regulated in several scenarios affecting source-sink relationships, such as the cytokinin-induced delay of senescence [35,36]. However, they appear to have a dual role, being involved also in stimulating the cell cycle through the production of sugar signals, the latter implicating them, along with the cytokinins, in cell division and seed development [37,38,39,40]. Cell wall invertases are likely also to be involved in the mobilisation of resources during seed germination.
The cytokinins are clearly implicated in seed development [41]. The cytokinins are biosynthesised by isopentenyl transferase (IPT), degraded by cytokinin oxidase/dehydrogenase (CKX), and conjugated to storage or inactivated forms by glycosidases. The first formed cytokinins are nucleotides, which may be activated to the free base forms by LONELY GUY (LOG) [42]. A signal transduction pathway is activated upon the detection of free base cytokinins by receptors [43], which activate response regulators (RR) downstream (for recent reviews, see [44,45]).
The feeding of radioactively-labelled precursors has shown that germinating lupin and maize seeds are capable of biosynthesising cytokinin, but that this is restricted to the embryo axis [46,47,48,49]. In maize, the cytokinin then moves unidirectionally from the embryo to accumulate in the endosperm in maize [48]. The cytokinins synthesized by the embryonic axis of lupin, and which are also transported unidirectionally to the cotyledons [46], were shown to be highly stable and to induce cotyledon expansion and chlorophyll synthesis [47]. Further, the regulation of reserve mobilization in yellow lupin seeds and in germinating chick-peas, appears to be mediated, at least in part, by cytokinin emanating from the embryonic axis [49,50]. Differential activity of different cytokinin forms has also been suggested in germinating chick-pea with zeatin riboside (ZR) affecting the mobilisation of carbohydrate, whereas zeatin (Z) had more impact on the protein in the cotyledons; isopentenyl adenine (iP) affected only the metabolism of carbohydrates, whereas iPR (iP riboside) mainly affected lipid metabolism [50,51]. Interestingly, all enzymes of the isoprenoid pathway have increased in activity within 2 to 6 h from the start of imbibition [52], providing precursors to the cytokinin biosynthetic machinery.
As transporter genes (reviewed in [10,53]), and cytokinin biosynthetic and degradative genes (reviewed in [41]) are the targets of transgenic approaches to increasing seed quality and/or quantity, we were interested if these gene families were expressed during germination. Such knowledge is important as changes in the expression of genes in the parental generation may impact seed germination and seedling vigour of the subsequent generation.
We chose to work with pea as it is both an important legume crop and a well-studied model crop. Additionally, pea seeds are non-dormant and non-endospermous, and their covering layers are not a mechanical constraint to radical protrusion [54]. The germination process is divided into an initial rapid imbibition phase during which pea seeds exhibit increased respiration and metabolic activity [55], followed by an activation phase during which stored carbohydrate and protein are mobilised [56]. Our aim was to determine if the gene families known to be involved in sink activity during seed development (SWEETs, SUTs, CWINVs, IPT, LOG, CKX) are also involved in mobilising reserves during germination. Based on gene family members detected in our pea transcriptome [17], we used RT-qPCR to monitor their expression. We show that genes actively expressed when the seed is a strong sink during its development are also actively expressed when the seed is in the reverse role of being an active source during germination.
2. Results
Expression relative to the reference genes is shown for all gene family members at four hours post-imbibition (4 hpi) (Figure 1). This timing coincides with Phase I water uptake during imbibition by peas and is prior to mass reserve mobilisation [52]. The data for subsequent time points are shown in a heat map as fold-change relative to 4 hpi (Figure 2).
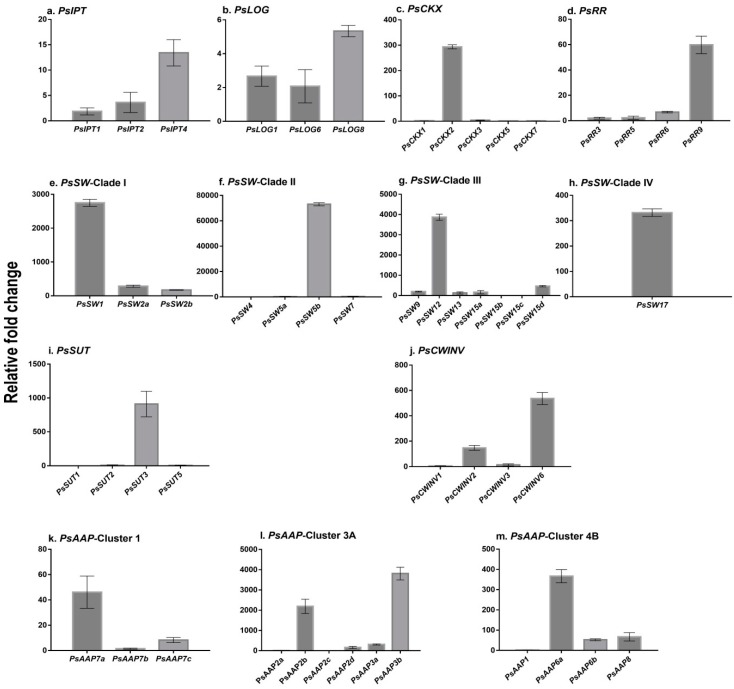
Figure 1.
Relative expression of cytokinin biosynthesis (PsIPT), activation (PsLOG), degradation (PsCKX) and response regulator (PsRR) gene family members along with PsSWEET (PsSW), PsSUT, PsCWINV and PsAAP gene family members in Pisum sativum cotyledons after four hours of imbibition. Fold-change values were calculated using PsEF, U18S, PsGAP and PsACT as internal controls using three technical replicates for each of two biological replicates in the RT-qPCR. The results are expressed as ± SD.
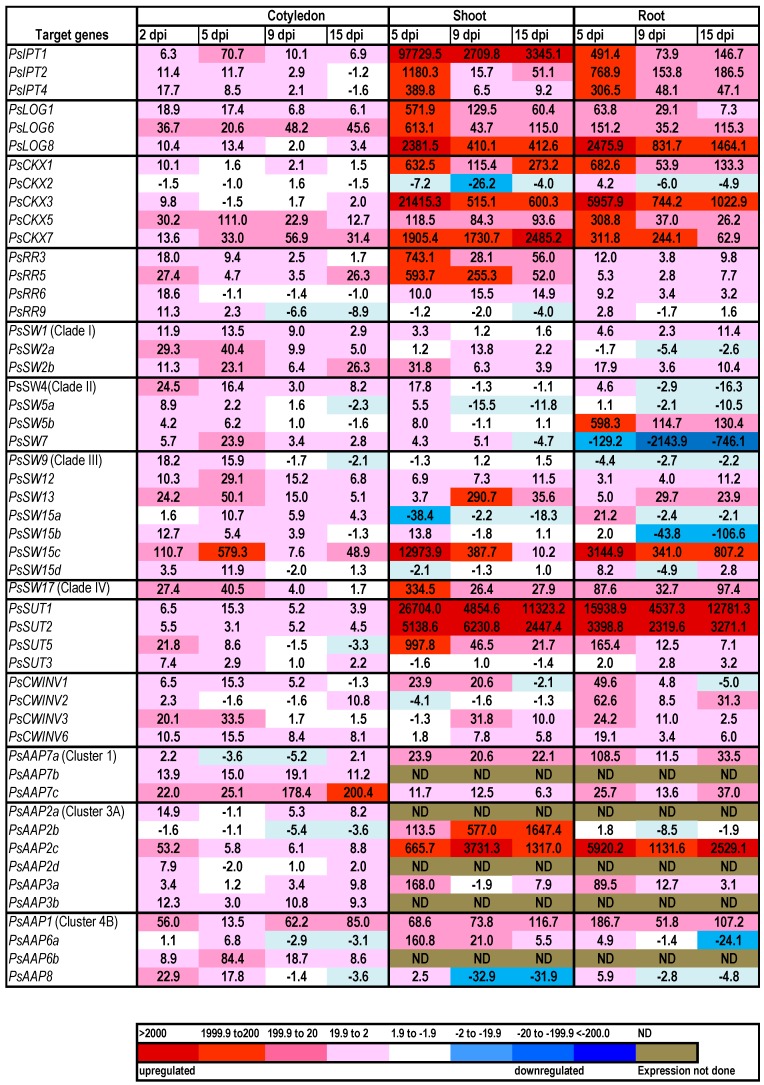
Figure 2.
Relative expression of cytokinin biosynthesis (PsIPT), activation (PsLOG) degradation (PsCKX) and response regulator (PsRR) gene family members along with PsSWEET, PsSUT, PsCWINV and PsAAP gene family members in Pisum sativum cotyledons at two days post-imbibition (dpi), and in cotyledons, shoots and roots at 5, 9 and 15 dpi. Values are fold-changes relative to the expression at four hours post-imbibition (4 hpi). The colour scale indicates up-regulated expression (red scale), similar (white) and down-regulated expression (blue scale) relative to 4 hpi.
2.1. Cytokinin Biosynthesis and Metabolism in the Germinating Pea
The three PsIPT gene family members identified from the transcriptome were expressed in cotyledons at 4 hpi (Figure 1). Expression of the three PsIPT gene family members increased in cotyledons as they germinated, but reduced at later stages. Expression of PsIPT was at its greatest in the emerging roots and shoots (Figure 2). The three LOG family members expressed in cotyledons, roots and shoots. Expression in the cotyledons at 4 hpi was low (Figure 1) relative to later stages of germination (Figure 2). Relative to 4 hpi, the PsLOGs were expressed in the developing shoots and the elongating roots, particularly PsLOG8 (Figure 2).
Relative to the other three PsCKX family members, PsCKX2 was more strongly expressed within 4 hpi (Figure 1). This level changed little in the germinating cotyledon (hence, showing as “no change” on the heat map (Figure 2)). By 2 dpi, PsCKX5 and 7 had increased in the cotyledon. PsCKX2 remained constitutively expressed as roots and shoots developed, whereas PsCKX1, 3, 5 and 7 increased substantially in the developing shoots and roots (Figure 2). Of the four response regulators, which are putative Type As [17], PsRR9 was expressed within 4 hpi. All family members showed increased expression in cotyledons at 2 dpi relative to 4 hpi. PsRR3 and 5 were strongly expressed in the developing shoot, but much less strongly expressed in the elongating root (Figure 2). In imbibing seeds at 4 hpi, only cytokinin free bases and ribosides were detected, including both trans zeatin (tZ) and isopentenyladenine (iP) [17], but much lesser levels of dihydrozeatin (DZ) and cZ (Table 1). No O- or N-glucosides (Table 1) nor nucleotides ([17]; Table 1) were detected at 4 hpi. Two days post-imbibition, the cytokinin levels had increased substantially, to some extent due to an increase in tZ, but mostly due to the more substantial increase in nucleotides, particularly iPRMP (iP ribosyl monophosphate) [17] and, to a lesser extent, cis zeatin riboside monophosphate (cZRMP) and tZRMP. Cytokinin levels in the cotyledons peaked at 11 days and then declined, due to a decrease in nucleotides ([17]; Table 1). Developing shoots, first analysed five days after imbibition, had the greatest amount of cytokinin relative to later stages and predominantly as nucleotides with iPRMP ≅ cZRMP >>tZRMP (Table 1). Emerging roots at five days post-imbibition, had less cytokinin then shoots, but more than cotyledons on a DW basis. In contrast to shoots, the cytokinin level in roots increased to a peak at 11 dpi, and then declined. Again the major contributors to the cytokinin content were the nucleotides with iPRMP ≅ cZRMP > tZRMP, with free bases and ribosides contributing to a lesser extent (Table 1). Cytokinin O-glucosides were not detected at 4 hpi, but accumulated over time in cotyledons, shoots and roots. Zeatin 9-glucosides accumulated to low levels in seedling roots and shoots (Table 1), but no 7-glucosides were detected.
Table 1.
Endogenous cytokinins in germinating pea seeds. The data are the averages of four biological replicates and are expressed as ± SD.
| Cytokinin Levels | Cotyledon | Shoot | Root | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (pmol/g DW) | 4 hpi | 2 dpi | 5 dpi | 11 dpi | 15 dpi | 25 dpi | 5 dpi | 11 dpi | 15 dpi | 25 dpi | 5 dpi | 11 dpi | 15 dpi | 25 dpi |
| Total cytokinin | 5.05 ± 0.4 | 38.5 ± 7.3 | 51.37 ± 4.4 | 77.18 ± 11.4 | 47.23 ± 2.2 | 28.65 ± 2.2 | 357.41 ± 17.3 | 93.97 ± 14.9 | 55.83 ± 2.4 | 43.68 ± 1.3 | 197.89 ± 14.4 | 220.5 ± 26.8 | 113.68 ± 9.1 | 94.11 ± 5.9 |
| Total nucleotides | <lod | 34.5 ± 1.9 | 43.13 ± 3.8 | 64.73 ± 10.5 | 20.29 ± 2.8 | 9.39 ± 1.8 | 289.8 ± 13.6 | 65.44 ± 11.3 | 30.05 ± 2.8 | 17.64 ± 0.9 | 134.03 ± 13.3 | 162.86 ± 24.3 | 47.96 ± 5.0 | 31.61 ± 5.8 |
| Total bases | 3.72 ± 0.3 | 7.27 ± 0.6 | 3.72 ± 0.3 | 5.55 ± 0.5 | 2.75 ± 0.9 | 4.11 ± 0.3 | 17.31 ± 1.5 | 7.29 ± 1.1 | 5.9 ± 1.5 | 6.31 ± 0.5 | 12.65 ± 1.2 | 10.62 ± 0.3 | 8.97 ± 1.6 | 10.39 ± 0.5 |
| Total ribosides | 1.33 ± 0.1 | 3.56 ± 0.4 | 2.57 ± 0.4 | 3.07 ± 0.3 | 3.94 ± 0.4 | 2.36 ± 0.4 | 32.38 ± 3.3 | 8.36 ± 1.6 | 7.16 ± 0.5 | 3.71 ± 0.5 | 33.42 ± 2.0 | 26.24 ± 1.9 | 37 ± 8.2 | 13.87 ± 2.5 |
| Total O-glucosides | <lod | 2.08 ± 0.3 | 1.95 ± 0.2 | 3.75 ± 0.9 | 19.68 ± 0.9 | 11.81 ± 2.2 | 17.92 ± 1.5 | <lod | 12.49 ± 1.3 | 15.04 ± 1.8 | 17.48 ± 1.6 | 19.58 ± 1.3 | 19.03 ± 2.9 | 34.56 ± 4.2 |
| Total N-glucosides | <lod | <lod | <lod | 0.07 ± 0.02 | 0.64 ± 0.2 | 1.13 ± 0.4 | <lod | <lod | 0.24 ± 0.1 | 0.97 ± 0.1 | 0.34 ± 0.02 | 1.2 ± 0.3 | 0.73 ± 0.1 | 3.68 ± 0.4 |
| tZRMP | - | - | - | - | - | - | 23.87 ± 2.1 | 11.41 ± 1.8 | 2.19 ± 0.3 | 0.61 ± 0.04 | 15.17 ± 1.9 | 37.66 ± 6.9 | 6.68 ± 1.8 | 1.27 ± 0.4 |
| DHZRMP | <lod | <lod | <lod | 0.1 ± 0.01 | <lod | 0.27 ± 0.1 | 3.18 ± 0.1 | 0.66 ± 0.1 | 1.05 ± 0.3 | 0.89 ± 0.1 | 1.61 ± 0.3 | 0.83 ± 0.2 | 0.58 ± 0.1 | 0.26 ± 0.1 |
| iPRMP | - | - | - | - | - | - | 142.08 ± 9.9 | 22.66 ± 4.5 | 10.76 ± 1.3 | 6.91 ± 0.5 | 69 ± 4.02 | 68.53 ± 15.6 | 16.32 ± 1.0 | 10.09 ± 3.0 |
| cZRMP | <lod | 10.4 ± 1.2 | 17.25 ± 2.7 | 43 ± 9.1 | 15.07 ± 1.8 | 6.34 ± 1.1 | 120.66 ± 3.9 | 30.98 ± 6.6 | 17.76 ± 0.8 | 9.23 ± 0.5 | 48.25 ± 10.8 | 55.83 ± 9.0 | 24.46 ± 3.03 | 19.99 ± 3.1 |
| tZ | - | - | - | - | - | - | 7.16 ± 0.6 | 3.81 ± 0.8 | 1.74 ± 0.1 | 1.74 ± 0.1 | 4.55 ± 0.5 | 5.75 ± 0.5 | 3.83 ± 1.0 | 1.33 ± 0.1 |
| DHZ | 0.24 ± 0.04 | 0.1 ± 0.02 | 0.04 ± 0.01 | <lod | 0.09 ± 0.01 | 0.22 ± 0.1 | 0.49 ± 0.1 | <lod | 0.77 ± 0.1 | 1.26 ± 0.1 | 0.29 ± 0.1 | <lod | 0.41 ± 0.1 | 0.38 ± 0.02 |
| iP | - | - | - | - | - | - | 7.34 ± 0.7 | 2.79 ± 1.0 | 2.63 ± 1.0 | 2.41 ± 0.3 | 5.82 ± 0.8 | 3.62 ± 0.2 | 3.53 ± 0.6 | 3.22 ± 0.4 |
| cZ | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.19 ± 0.02 | 1.01 ± 0.2 | 0.62 ± 0.1 | 0.68 ± 0.1 | 2.31 ± 0.3 | 0.69 ± 0.1 | 0.76 ± 0.1 | 0.91 ± 0.1 | 2.1 ± 0.2 | 1.25 ± 0.2 | 1.2 ± 0.1 | 5.46 ± 0.9 |
| tZR | - | - | - | - | - | - | 4.01 ± 0.3 | 1.86 ± 0.3 | 0.47 ± 0.1 | 0.28 ± 0.1 | 6.78 ± 0.4 | 9.13 ± 0.7 | 6.21 ± 1.4 | 0.75 ± 0.2 |
| DHZR | 0.17 ± 0.1 | 0.39 ± 0.1 | 0.15 ± 0.02 | 0.2 ± 0.03 | 0.12 ± 0.0 | 0.18 ± 0.04 | 2.85 ± 0.3 | 1.65 ± 0.4 | 0.69 ± 0.1 | 0.63 ± 0.1 | 2.18 ± 0.1 | 2.28 ± 0.2 | 0.85 ± 0.2 | 0.8 ± 0.2 |
| iPR | - | - | - | - | - | - | 13.12 ± 2.5 | 2.88 ± 0.8 | 2.98 ± 0.8 | 1.33 ± 0.1 | 19.19 ± 1.7 | 11.24 ± 1.9 | 24.44 ± 5.8 | 7.81 ± 2.0 |
| cZR | 0.89 ± 0.1 | 0.65 ± 0.03 | 0.77 ± 0.1 | 1.57 ± 0.2 | 2.66 ± 0.5 | 1.19 ± 0.3 | 12.41 ± 2.3 | 1.97 ± 0.3 | 3.02 ± 0.8 | 1.47 ± 0.3 | 5.27 ± 0.1 | 3.59 ± 0.5 | 5.5 ± 1.0 | 4.51 ± 0.3 |
| tZOG | - | - | - | - | - | - | 1.42 ± 0.14 | 4.03 ± 0.8 | 1.74 ± 0.14 | 1.5 ± 0.1 | 0.73 ± 0.1 | 4.04 ± 0.6 | 1.94 ± 0.2 | 0.9 ± 0.1 |
| DHZOG | <lod | <lod | 0.03 ± 0.0 | 0.15 ± 0.02 | 0.3 ± 0.03 | 0.31 ± 0.04 | 0.32 ± 0.04 | 1.25 ± 0.2 | 2.71 ± 0.3 | 6.79 ± 1.1 | 0.19 ± 0.01 | 1.41 ± 0.1 | 1.05 ± 0.1 | 0.34 ± 0.03 |
| cZOG | <lod | 1.26 ± 0.1 | 0.98 ± 0.1 | 1.76 ± 0.4 | 9.63 ± 0.3 | 4.45 ± 0.8 | 2.54 ± 0.4 | 2.52 ± 0.4 | 2.93 ± 0.2 | 3.15 ± 0.3 | 2.83 ± 0.2 | 3.54 ± 0.6 | 2.24 ± 0.2 | 3.77 ± 0.5 |
| tZROG | - | - | - | - | - | - | 1.35 ± 0.1 | 1.25 ± 0.2 | 1.05 ± 0.1 | 1.03 ± 0.1 | 0.7 ± 0.03 | 1.12 ± 0.2 | 1.3 ± 0.32 | 0.7 ± 0.1 |
| DHZROG | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod |
| cZROG | <lod | 0.74 ± 0.2 | 0.66 ± 0.1 | 1.29 ± 0.4 | 8.39 ± 0.7 | 6.22 ± 1.6 | 12.3 ± 1.5 | 3.84 ± 0.5 | 4.05 ± 0.8 | 2.58 ± 0.3 | 13.04 ± 1.4 | 9.48 ± 0.8 | 12.5 ± 2.9 | 28.85 ± 3.9 |
| tZ9G | <lod | <lod | <lod | 0.12 ± 0.02 | 0.64 ± 0.2 | 1.13 ± 0.4 | <lod | <lod | 0.24 ± 0.1 | 0.97 ± 0.1 | <lod | <lod | 0.39 ± 0.1 | 2.88 ± 0.3 |
| DHZ9G | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod |
| iP9G | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod |
| cZ9G | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | <lod | 0.34 ± 0.02 | 0.24 ± 0.01 | 0.34 ± 0.1 | 0.81 ± 0.1 |
lod, below the limits of detection; - indicates the values presented in Dhandapani et al. (2017).
2.2. Expression of Transporter Genes in the Germinating Seed
The relative expression of the PsSWEET gene family members differed in the germinating seeds within 4 hpi with, for example, extremely low values (<5) for Clade III PsSW15b and 15c and extremely high values (ca. 70,000) for Clade II PsSW5b (Figure 1). Generally, the PsSWEETs increased in expression in the cotyledons following imbibition. PsSWEET1, 5b and 12 were generally constitutively expressed in the developing shoots and roots. PsSWEET15c was strongly expressed in the younger developing shoots and roots. Clade IV PsSWEET17 was elevated in expression in cotyledons, shoots and roots (Figure 2).
Type II PsSUT3 was elevated relative to the three Type I SUTs in the imbibing seeds at 4 hpi (Figure 1), and was subsequently more-or-less constitutively expressed (Figure 2). In the cotyledons, there was generally an increase in the expression of the other PsSUTs as the seeds germinated (Figure 2). However, relative to the expression at 4 hpi, both PsSUT1 and 2 were very strongly expressed in both developing shoots and roots.
The four PsCWINV gene family members were expressed in the imbibing seeds, particularly PsCWINV6 (Figure 1), with expression generally increasing in the cotyledons with germination. Expression was more consistently elevated in roots than in shoots (Figure 2).
Most of the 13 PsAAP gene family members were expressing in cotyledons within 4 hpi, with two Cluster 3A gene family members strongly expressed (Figure 1). There was an increase in expression of the 13 PsAAP gene family members in germinating seeds, generally peaking at 2 dpi or 5 dpi in the cotyledons (Figure 2). Strong expression of PsAAP2c was evident in both roots and shoots, and of 2b in shoots. Lower level but consistent expression was apparent for PsAAP3a (Cluster 3A) and PsAAP1 and 6a (Cluster 4B) across organs. PsAAP7 expressed increasingly strongly over time, whereas PsAAP8 showed low and decreasing expression over time.
3. Discussion
Germinating seeds are metabolically highly active, exhibiting increased respiration and metabolic activity within a few hours of imbibition, followed by the degradation of carbohydrate, lipid and protein stores, and the mobilisation of these to the embryo [57,58]. Pea stores both carbohydrate and protein that must be metabolised and transported from the cotyledons to the embryo axis and thence to the elongating root and shoot. Cytokinins and expression of SWEETs, SUTs, CWINVs and AAPs were all detected within four hours of commencement of imbibition. Within two days of imbibition, at which stage the radicle and plumule had emerged, increased expression of most gene families was occurring.
Within 4 hpi, expression of PsIPT was detected as were low levels of biologically active cytokinins. As the seeds germinated, the accumulation of cytokinin nucleotides was strong evidence of cytokinin biosynthesis occurring [59], and the elevated expression of response regulators indicated that the cytokinin signal transduction pathway was operational [36]. This strongly supports the contention that cytokinin emanating from the embryonic axis of legumes is biosynthesised in situ and is involved in early reserve mobilisation [49,51]. As roots and shoots emerged, increased cytokinin biosynthesis was apparent. The increase in nucleotides as the seeds germinated is similar to that reported for germinating Tagetes minuta L. [60]. Earlier work with chick-peas and more recent work with pea did not report nucleotide levels [50,56] but these appear to be the most significant cytokinin form in the germinating seed and during early seedling growth ([17]; Table 1). The origin of the cZRMP is of interest. It has yet to be determined whether the cytokinin released from tRNA (the usually cited source of the cis cytokinins) has been phosphorylated, making it then accessible to LOG, or whether it has been directly biosynthesised.
The active forms of the cytokinins are the free bases [43] which are present at low levels in the imbibing seed [17] and are particularly evident in the emerging shoot and root (Table 1). Several of the earlier papers [46,50,60] refer to the dihydro-derivatives, which are not metabolised by CKX. DHZRMP, DHZ and DHZR were detected in pea but only at low levels in the cotyledons and in the emerging shoots and roots. Cytokinin biosynthesis (as determined by expression of PsIPT and the levels of nucleotides) was more strongly elevated in early shoot growth compared with the roots. Most notable though were the elevated levels of the RR genes in shoots compared with roots, indicating a stronger response to the cytokinin in the shoots compared with the roots. This aligns with our knowledge that cytokinin is known to promote shoot growth and inhibit aspects of root growth [61].
It is important to note that CKX expression was also increasing during germination, and was strongly upregulated in the young seedling shoots and roots. An increase in CKX at the time when cytokinin levels are increasing is a common phenomenon, and is indicative of homeostatic mechanisms operating [41,45]. CKX activity is the likely reason for the levels of free bases and ribosides being significantly less than those of the nucleotides: strong biosynthesis and metabolism of cytokinin are occurring during early seedling growth.
As decreasing CKX activity is a target for enhancing yield in both monocots and dicots [41,62,63] it is important to be aware that any increased cytokinin may impact source-sink relationships. Particularly critical in peas may be PsCKX2, which is expressed in the imbibing seed: if this gene family member were to be down-regulated, this may impact severely on the release of nutrients from the cotyledon. Down-regulation of PsCKX7 may have a double impact on the root by stimulating competitive sink activity in the shoot, as well as inhibiting root growth through elevated endogenous cytokinin [61].
Transgenic work has implicated both a Clade II and a Clade IV SWEET in the germination of Arabidopsis seeds [31,32]. We show here that several PsSWEET gene family members are strongly expressed in germinating pea seeds with PsSWEET5b (a Clade II SWEET) very strongly expressed within 4 hpi and PsSW17 (a Clade IV SWEET) strongly up-regulated during germination.
Elevated expression of PsCWINV gene family members occurred during germination and early seedling growth, again supporting a strong link between both cytokinin and CWINVs [35,38,39,40], and INV and SWEETs [12], with the likelihood of CWINVs converting sucrose to hexoses, available to Clade II SWEETs. Subsequently, Clade III SWEETs were also activated to move sucrose towards SUTs for uploading into the phloem. PsSUT3 appears to be more-or-less constitutively expressed, but both PsSUT1 and 2 are very strongly unregulated in the elongating root and shoot, particularly compared to their activity in the cotyledons.
The pea homologues of the AtAAP gene family members linked to seed loading in Arabidopsis (Cluster 4B, AtAAP1 and 8) [64,65] were not particularly strongly expressed in the imbibing pea seed. However, PsAAP1 was strongly upregulated in the cotyledons, shoots and roots as the seed germinated. Recently, Santiago and Tegeder (2016) suggested that AtAAP8 was the “long sought after phloem loader”, showing it capable of loading a broad spectrum of amino acids into the phloem [7]. Interestingly, this gene family member was not strongly expressed during germination, relative to some other AtAAP gene family members, and was barely expressed during early pod and seed development in pea (our unpublished data). Indeed, PsAAP8 was significantly down-regulated in the elongating shoots, in contrast to Cluster 3A member, PsAAP2b, which was specifically up-regulated in the shoot compared to the roots. PsAAP2c was the most highly expressed AAP gene family member in both the elongating roots and shoots, indicating clear differential expression of the PsAAP gene family members.
While targeting of nutrient transporters has led to increased yield in some instances [53], the impact of that manipulation has not been reported on the germinating seed. In this work we show that key gene families involved in seed development, some of which have been the targets of genetic manipulation, are also involved in germination. This information is important, as potential imbalances in the mobilisation of carbon and nitrogen resources may have detrimental effects on the developing seedling, as shown for Arabidopsis overexpressing SWEET16 under nitrogen-limiting conditions [31].
4. Materials and Methods
4.1. Plant Material and Sample Preparation
Surface sterilised seeds of Pisum sativum variety Bohatyr were imbibed for 4 h with stirring in Klambt medium [66] and placed in sterilised 500 mL containers with 0.6% (w/v) agar and 10% (w/v) Hoagland’s mineral salts solution [67]. The containers were placed in a growth room at 22 °C with a 16-h photoperiod. Five imbibed or germinating seeds/seedlings were sampled at 4 h, 2 days, 5 days, 9 days and 15 days after imbibition, submerged briefly in liquid nitrogen and stored at −80 °C until use.
4.2. RNA Isolation and Target Gene Isolation
Total RNA was extracted from each sample using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and converted to cDNA as previously described [17]. Sequences of candidate gene family members were isolated from an RNA-seq transcriptomic data set as described in [17]. Primers were designed for the RT-qPCR and their products sequenced. The primers used are reported in [17].
4.3. Real-Time Reverse Transcription Quantitative PCR (RT-qPCR)
The relative expression levels of each of the genes of interest were determined using RT-qPCR as described in [68], with two biological replicates and three technical replicates for each sample. PCR was performed in a Rotor-Gene Q (Qiagen, Hilden, Germany), with either a home-made SYBR Green master mix or the KAPA SYBR® FAST qPCR Kits (Kapa Biosystems, Boston, MA, USA). Four reference genes, PsEF, PsGAP, PsACT and U18S were used to correct the Ct values before calculating the relative expression of each sample as described in [68].
4.4. Cytokinin Analyses
Cotyledons from four individual imbibed seeds or seedlings, making four biological replicates, were ground under liquid nitrogen and freeze-dried. Cytokinins were extracted and purified as described in [17,69] and subjected to analysis by an LC-MS/MS system consisting of an ACQUITY UPLC® System (Waters, Milford, MA, USA) and Xevo® TQ-S (Waters) triple quadrupole mass spectrometer. Quantification was obtained using multiple reaction-monitoring (MRM) mode of selected precursor ions along with stable isotope internal standards [69].
Acknowledgments
The project funding and partial funding for Paula E. Jameson and Jiancheng Song came from the FRST/MBIE Advanced Seed Production Systems grant to Paula E. Jameson. Pragatheswari Dhandapani was funded by a University of Canterbury Doctoral Scholarship. Ondrej Novak was funded by the Ministry of Education, Youth and Sports of the Czech Republic (the National Program for Sustainability I (No. LO1204) and the "Návrat" program (LK21306)). Thanks to Matt Walters for finalising the figures.
Author Contributions
Paula E. Jameson was responsible for planning the project. Pragatheswari Dhandapani performed the experiments and analysed the RT-qPCR data. Jiancheng Song analysed the pea transcriptome and provided all bioinformatics input. Ondrej Novak conducted the cytokinin analyses and provided the cytokinin data. Paula E. Jameson wrote the paper, with input from all authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Yu S.-M., Lo S.-F., Ho T.-H.D. Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015;20:844–857. doi: 10.1016/j.tplants.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Roche J., Love J., Guo Q., Song J., Cao M., Fraser K., Huege J., Jones C., Novák O., Turnbull M.H., et al. Metabolic changes and associated cytokinin signals in response to nitrate assimilation in roots and shoots of Lolium perenne. Physiol. Plant. 2016;156:497–511. doi: 10.1111/ppl.12412. [DOI] [PubMed] [Google Scholar]
- 3.Wang L., Ruan Y.-L. Shoot–root carbon allocation, sugar signaling and their coupling with nitrogen uptake and assimilation. Funct. Plant Biol. 2016;43:105–113. doi: 10.1071/FP15249. [DOI] [PubMed] [Google Scholar]
- 4.Braun D.M., Wang L., Ruan Y.-L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014;65:1713–1735. doi: 10.1093/jxb/ert416. [DOI] [PubMed] [Google Scholar]
- 5.Ruan Y.-L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014;65:33–67. doi: 10.1146/annurev-arplant-050213-040251. [DOI] [PubMed] [Google Scholar]
- 6.Tegeder M. Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opin. Plant Biol. 2012;15:315–321. doi: 10.1016/j.pbi.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Santiago J.P., Tegeder M. Connecting source with sink: The role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol. 2016;171:508–521. doi: 10.1104/pp.16.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrick J.W., Offler C.E. Compartmentation of transport and transfer events in developing seeds. J. Exp. Bot. 2001;52:551–564. doi: 10.1093/jexbot/52.356.551. [DOI] [PubMed] [Google Scholar]
- 9.Rosche E., Blackmore D., Tegeder M., Richardson T., Schroeder H., Higgins T.J.V., Frommer W.B., Offler C.E., Patrick J.W. Seed-specific overexpression of a potato sucrose transporter increases sucrose uptake and growth rates of developing cotyledons. Plant J. 2002;30:165–175. doi: 10.1046/j.1365-313X.2002.01282.x. [DOI] [PubMed] [Google Scholar]
- 10.Tegeder M. Transporters involved in source to sink partitioning of amino acids and ureides: Opportunities for crop improvement. J. Exp. Bot. 2014;65:1865–1878. doi: 10.1093/jxb/eru012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Garneau M.G., Majumdar R., Grant J., Tegeder M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J. 2015;81:134–146. doi: 10.1111/tpj.12716. [DOI] [PubMed] [Google Scholar]
- 12.Eom J.S., Chen L.-Q., Sosso D., Benjamin T.J., Lin I.W., Qu X.-Q., Braun D.M., Frommer W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015;25:53–62. doi: 10.1016/j.pbi.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Tegeder M., Ward J.M. Molecular evolution of plant AAP and LHT amino acid transporters. Front. Plant Sci. 2012;3:21. doi: 10.3389/fpls.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beevers L., Guernsey F.S. Changes in some nitrogenous components during the germination of pea seeds. Plant Physiol. 1966;41:1455–1458. doi: 10.1104/pp.41.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrandt T.M., Nesi A.N., Araújo W.L., Braun H.-P. Amino acid catabolism in plants. Mol. Plant. 2015;8:1563–1579. doi: 10.1016/j.molp.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Taylor M.R., Reinders A., Ward J.M. Transport function of rice amino acid permeases (AAPs) Plant Cell Physiol. 2015;56:1355–1363. doi: 10.1093/pcp/pcv053. [DOI] [PubMed] [Google Scholar]
- 17.Dhandapani P., Song J., Novak O., Jameson P.E. Infection by Rhodococcus fascians maintains cotyledons as a sink tissue for the pathogen. Ann. Bot. 2016 doi: 10.1093/aob/mcw202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng D., Gu X., Xue L.-J., Leebens-Mack J.H., Tsai C.-J. Bayesian phylogeny of sucrose transporters: Ancient origins, differential expansion and convergent evolution in monocots and dicots. Front. Plant Sci. 2014;5:615. doi: 10.3389/fpls.2014.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y., Qu H., Dibley K.E., Offler C.E. A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J. 2007;49:750–764. doi: 10.1111/j.1365-313X.2006.03000.x. [DOI] [PubMed] [Google Scholar]
- 20.Reinders A., Sivitz A.B., Ward J.M. Evolution of plant sucrose uptake transporters. Front. Plant Sci. 2012;3:22. doi: 10.3389/fpls.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scofield G.N., Aoki N., Hirose T., Takano M., Jenkins C.L., Furbank R.T. The role of the sucrose transporter, OsSUT1, in germination and early seedling growth and development of rice plants. J. Exp. Bot. 2007;58:483–495. doi: 10.1093/jxb/erl217. [DOI] [PubMed] [Google Scholar]
- 22.Chung P., Hsiao H.-H., Chen H.-J., Chang C.-W., Wang S.-J. Influence of temperature on the expression of the rice sucrose transporter 4 gene, OsSUT4, in germinating embryos and maturing pollen. Acta Physiol. Plant. 2014;36:217–229. doi: 10.1007/s11738-013-1403-x. [DOI] [Google Scholar]
- 23.Slewinski T.L., Meeley R., Braun D.M. Sucrose transporter 1 functions in phloem loading in maize leaves. J. Exp. Bot. 2009;60:881–892. doi: 10.1093/jxb/ern335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bick J.A., Neelam A., Smith E., Nelson S.J., Hall J.L., Williams L.E. Expression analysis of a sucrose carrier in the germinating seedling of Ricinus communis. Plant Mol. Biol. 1998;38:425–435. doi: 10.1023/A:1006040306581. [DOI] [PubMed] [Google Scholar]
- 25.Tegeder M., Wang X.D., Frommer W.B., Offler C.E., Patrick J.W. Sucrose transport into developing seeds of Pisum sativum L. Plant J. 1999;18:151–161. doi: 10.1046/j.1365-313X.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- 26.Weber H., Borisjuk L., Wobus U. Sugar import and metabolism during seed development. Trends Plant Sci. 1997;2:169–174. doi: 10.1016/S1360-1385(97)85222-3. [DOI] [Google Scholar]
- 27.Chen L.-Q., Qu X.Q., Hou B.H., Sosso D., Osorio S., Fernie A.R., Frommer W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335:207–211. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- 28.Chen H.-Y., Huh J.-H., Yu Y.-C., Ho L.-H., Chen L.-Q., Tholl D., Frommer W.B., Guo W.-J. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 2015;83:1046–1058. doi: 10.1111/tpj.12948. [DOI] [PubMed] [Google Scholar]
- 29.Chandran D. Co-option of developmentally regulated plant SWEET transporters for pathogen nutrition and abiotic stress tolerance. IUBMB Life. 2015;67:461–471. doi: 10.1002/iub.1394. [DOI] [PubMed] [Google Scholar]
- 30.Patil G., Valliyodan B., Deshmukh R., Prince S., Nicander B., Zhao M., Sonah H., Song L., Lin L., Chaudhary J., et al. Soybean (Glycine max) SWEET gene family: Insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis. BMC Genom. 2015;16:520. doi: 10.1186/s12864-015-1730-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klemens P.A.W., Patzke K., Deitmer J., Spinner L., Le Hir R., Bellini C., Bedu M., Chardon F., Krapp A., Neuhaus H.E. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth and stress tolerance in Arabidopsis. Plant Physiol. 2013;163:1338–1352. doi: 10.1104/pp.113.224972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X., Zhang Y., Yang C., Tian Z., Li J. AtSWEET4, a hexose facilitator, mediates sugar transport to axial sinks and affects plant development. Sci. Rep. 2016;6 doi: 10.1038/srep24563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan Y.-L., Jin Y., Yang Y.-J., Li G.-J., Boyer J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant. 2010;3:942–955. doi: 10.1093/mp/ssq044. [DOI] [PubMed] [Google Scholar]
- 34.Roitsch T., Gonzalez M.C. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 2004;9:606–613. doi: 10.1016/j.tplants.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Balibrea Lara M.E., Gonzalez Garcia M.-C., Fatima T., Ehneß R., Lee T.K., Proels R., Tanner W., Roitsch T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell. 2004;16:1276–1287. doi: 10.1105/tpc.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang I., Sheen J., Muller B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012;63:353–380. doi: 10.1146/annurev-arplant-042811-105503. [DOI] [PubMed] [Google Scholar]
- 37.Cheng W.H., Taliercio E.W., Chourey P.S. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell. 1996;8:971–983. doi: 10.1105/tpc.8.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehneß R., Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11:539–548. doi: 10.1046/j.1365-313X.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- 39.Rijavec T., Kovač M., Kladnik A., Chourey P.S., Dermastia M. A comparative study on the role of cytokinins in caryopsis development in the maize miniature1 seed mutant and its wild type. J. Integr. Plant Biol. 2009;51:840–849. doi: 10.1111/j.1744-7909.2009.00863.x. [DOI] [PubMed] [Google Scholar]
- 40.Song J., Jiang L., Jameson P.E. Expression patterns of Brassica napus genes implicate IPT, CKX, sucrose transporter, cell wall invertase and amino acid permease gene family members in leaf, flower, silique and seed development. J. Exp. Bot. 2015;66:5067–5082. doi: 10.1093/jxb/erv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jameson P.E., Song J. Cytokinin: A key driver of seed yield. J. Exp. Bot. 2016;67:593–606. doi: 10.1093/jxb/erv461. [DOI] [PubMed] [Google Scholar]
- 42.Kuroha T., Tokunaga H., Kojima M., Ishida T., Nagawa S., Fukuda H., Sugimoto K., Sakakibara H. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct action pathway in Arabidopsis. Plant Cell. 2009;21:3152–3169. doi: 10.1105/tpc.109.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lomin S.N., Krivosheev D.M., Steklov M.Y., Arkhipov D.V., Osolodkin D.I., Schmülling T., Romanov G.A. Plant membrane assays with cytokinin receptors underpin the unique role of free cytokinin bases as biologically active ligands. J. Exp. Bot. 2015;66:1851–1863. doi: 10.1093/jxb/eru522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spichal L. Cytokinins—Recent news and views of evolutionally old molecules. Funct. Plant Biol. 2012;39:267–284. doi: 10.1071/FP11276. [DOI] [PubMed] [Google Scholar]
- 45.Jameson P.E. Cytokinins. In: Thomas B., Murray B.G., Murphy D.J., editors. Encyclopedia of Applied Plant Sciences. Volume 1. Academic Press; Waltham, MA, USA: 2017. pp. 391–402. [Google Scholar]
- 46.Nandi S.K., Palni L.M.S., Letham D.S., Knypl J.S. The biosynthesis of cytokinins in germinating lupin seeds. J. Exp. Bot. 1988;39:1649–1665. doi: 10.1093/jxb/39.12.1649. [DOI] [Google Scholar]
- 47.Nandi S.K., Palni L.M.S. Transport and metabolism of dihydrozeatin riboside in germinating lupin seeds. J. Exp. Bot. 1989;40:615–629. doi: 10.1093/jxb/40.5.615. [DOI] [Google Scholar]
- 48.Hocart C.H., Letham D.S. Biosynthesis of cytokinin in germinating seeds of Zea mays. J. Exp. Bot. 1990;41:1525–1528. doi: 10.1093/jxb/41.12.1525. [DOI] [Google Scholar]
- 49.Nandi S.K., Palni L.M.S., de Klerk G.J.M. The influence of the embryonic axis and cytokinins on reserve mobilization in germinating lupin seeds. J. Exp. Bot. 1995;46:329–336. doi: 10.1093/jxb/46.3.329. [DOI] [Google Scholar]
- 50.Villalobos N., Martin L. Involvement of cytokinins in the germination of chick-pea seeds. Plant Growth Regul. 1992;11:277–291. doi: 10.1007/BF00024567. [DOI] [Google Scholar]
- 51.Munoz J.L., Martin L., Nicolas G., Villalobos N. Influence of endogenous cytokinin on reserve mobilization in cotyledons of Cicer arietinum L. Plant Physiol. 1990;93:1011–1016. doi: 10.1104/pp.93.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green T.R., Baisted D.J. Development of the activities of enzymes of the isoprenoid pathway during early stages of pea-seed germination. Biochem. J. 1972;130:983–995. doi: 10.1042/bj1300983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yadav U.P., Ayre B.G., Bush D.R. Transgenic approaches to altering carbon and nitrogen partitioning in whole plants: Assessing the potential to improve crop yields and nutritional quality. Front. Plant Sci. 2015;6:275. doi: 10.3389/fpls.2015.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petruzzelli L., Kunz C., Waldvogel R., Meins F., Leubner-Metzger G. Distinct ethylene- and tissue-specific regulation of β-1,3-glucanases and chitinases during pea seed germination. Planta. 1999;209:195–201. doi: 10.1007/s004250050622. [DOI] [PubMed] [Google Scholar]
- 55.Nawa Y., Asahi T. Relationship between the water content of pea cotyledons and mitochondrial development during the early stage of germination. Plant Cell Physiol. 1973;14:607–610. [Google Scholar]
- 56.Barba-Espín G., Diaz-Vivancos P., Job D., Belghazi M., Job C., Hernández A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011;34:1907–1919. doi: 10.1111/j.1365-3040.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- 57.Bewley J.D. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weitbrecht K., Müller K., Leubner-Metzger G. First off the mark: Early seed germination. J. Exp. Bot. 2011;62:3289–3309. doi: 10.1093/jxb/err030. [DOI] [PubMed] [Google Scholar]
- 59.Hirose N., Takei K., Huroha T., Kamada-Nobusada T., Hayashi H., Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- 60.Stirk W.A., Gold J.D., Novák O., Strnad M., van Staden J. Changes in endogenous cytokinins during germination and seedling establishment of Tagetes minuta L. Plant Growth Regul. 2005;47:1–7. doi: 10.1007/s10725-005-1767-z. [DOI] [Google Scholar]
- 61.Werner T., Holst K., Pörs Y., Guivarc'h A., Mustroph A., Chriqui D., Grimm B., Schmülling T. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 2008;59:2659–2672. doi: 10.1093/jxb/ern134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashikari M., Sakakibara H., Lin S.Y., Yamamoto T., Takashi T., Nishimura A., Angeles E.R., Qian Q., Kitano H., Matsuoka M. Cytokinin oxidase regulates rice grain production. Science. 2005;309:741–745. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- 63.Zalewski W., Gasparis S., Boczkowska M., Rajchel I., Orczyk W., Nadolska-Orczyk A. Expression patterns of HvCKX genes indicate their role in growth and reproductive development of barley. PLoS ONE. 2014 doi: 10.1371/journal.pone.0115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanders A., Collier R., Trethewy A., Gould G., Sieker R., Tegeder M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009;59:540–552. doi: 10.1111/j.1365-313X.2009.03890.x. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt R., Stransky H., Koch W. The amino acid permease AAP8 is important for early seed development in Arabidopsis. Planta. 2007;226:805–813. doi: 10.1007/s00425-007-0527-x. [DOI] [PubMed] [Google Scholar]
- 66.Klambt D., Thies G., Skoog F. Isolation of cytokinins from Corynebacterium. fascians. Proc. Natl. Acad. Sci. USA. 1966;56:52–59. doi: 10.1073/pnas.56.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lawson E., Gantotti B., Starr M. A 78-megadalton plasmid occurs in avirulent strains as well as virulent strains of Corynebacterium fascians. Curr. Microbiol. 1982;7:327–332. doi: 10.1007/BF01572598. [DOI] [Google Scholar]
- 68.Song J., Jiang L., Jameson P.E. Co-ordinate regulation of cytokinin gene family members during flag leaf and reproductive development in wheat. BMC Plant Biol. 2012;12:78. doi: 10.1186/1471-2229-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antoniadi I., Plačková L., Simonovik B., Doležal K., Turnbull C., Ljung K., Novák O. Cell-type specific cytokinin distribution within the Arabidopsis primary root apex. Plant Cell. 2015;27:1955–1967. doi: 10.1105/tpc.15.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]