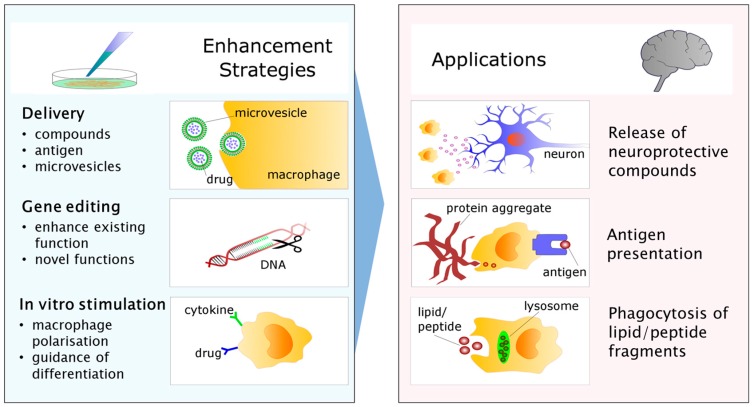
Figure 3.
Strategies for enhancing myeloid cell therapy for the CNS. The phagocytic capacity of myeloid cells may be adapted for use as delivery vehicles for compounds, antigens, or microvesicles loaded with therapeutic substances destined for the brain. Pictured is a macrophage in the process of taking up drug-loaded nanoparticles intended for delivery into the CNS. Gene editing enables the up/downregulation of existing functions to enhance protective, or eliminate damaging responses to disease. It is also possible to introduce expression of enzymes not usually present in these cells through this process. Another strategy for guiding myeloid cell-based therapies in the CNS involves in vitro manipulation of polarisation or differentiation, for example with cytokines or drugs known to guide the cells into a more neuroprotective phenotype. As a result of in vitro manipulation, myeloid cells returned to the circulation may then be better capable of ameliorating brain pathology by one of several mechanisms. For example, CNS-infiltrating myeloid cells may be better equipped to release compounds with growth or survival-promoting effects on nearby diseased neurons. Alternatively, it may be possible to enhance the capacity of infiltrating cells to present brain-derived antigens to the adaptive immune system, thus enhancing clearance of unwanted protein products. Myeloid cells may also be used to enhance the clearance of excess waste or diseased tissue independently via phagocytosis.