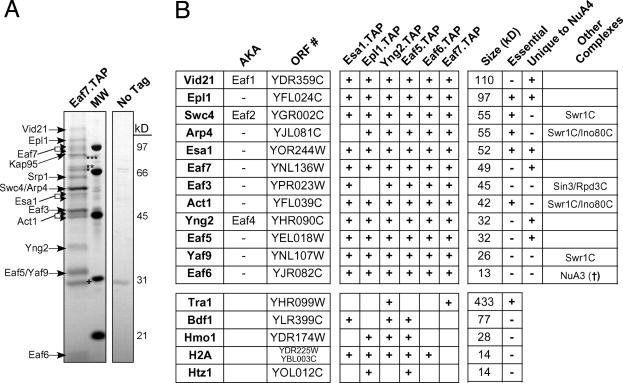
Fig. 1.
Isolation of the NuA4 HAT complex. (A) TAP of the NuA4 complex was carried out on strains containing either TAP-tagged Eaf7 or no tagged protein. The purified protein was analyzed by SDS/PAGE and silver staining. The indicated proteins were identified by trypsin digestion of each stained band followed by MALDI-TOF mass spectrometry or tandem mass spectrometry after subjecting an aliquot of the eluate from the final column directly to trypsin. Contaminating bands are indicated: ***, Sse1; **, Ssa1; *, Ssb1; †, TEV protease. The Srp1/Kap95 importin complex, which transports NLS-containing proteins into the nucleus (56), also copurified with NuA4 and is often found associated with nuclear protein complexes (N.J.K., A.E. and J.F.G., unpublished data). (B) Summary of purified proteins identified by mass spectrometry. Proteins that were present in at least two of the six purifications are represented. Protein size (kDa) was as predicted by amino acid composition (Saccharomyces Genome Database; www.yeastgenome.org) and not determined experimentally. (†, N.J.K., A.E., and J.F.G., unpublished data.)