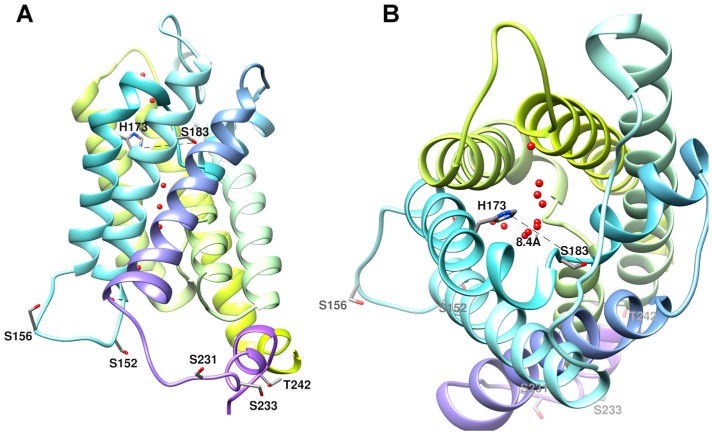
Figure 3.
Structure of human AQP5 monomer. (A) Side view of the monomer with several phosphorylation consensus sites in the cytoplasmic region (Ser152, Ser156, Ser231, Ser233, and Thr242) shown in licorice representation. Thr259, also a phosphorylation site, is not represented due to the inexistent electron density beyond Pro245 for hAQP5 structure [49]; (B) Top view of the monomer with His173 and Ser183 in the selectivity filter (SF) shown in licorice representation. The distance between these two residues (8.4 Å) corresponds to the proposed distance for the SF wide conformation [33]. Water molecules are shown as red spheres along the channel pore. Structures were generated with Chimera (http://www.cgl.ucsf.edu/chimera) and are based on AQP5 X-ray structure (PDB databank code 3D9S).