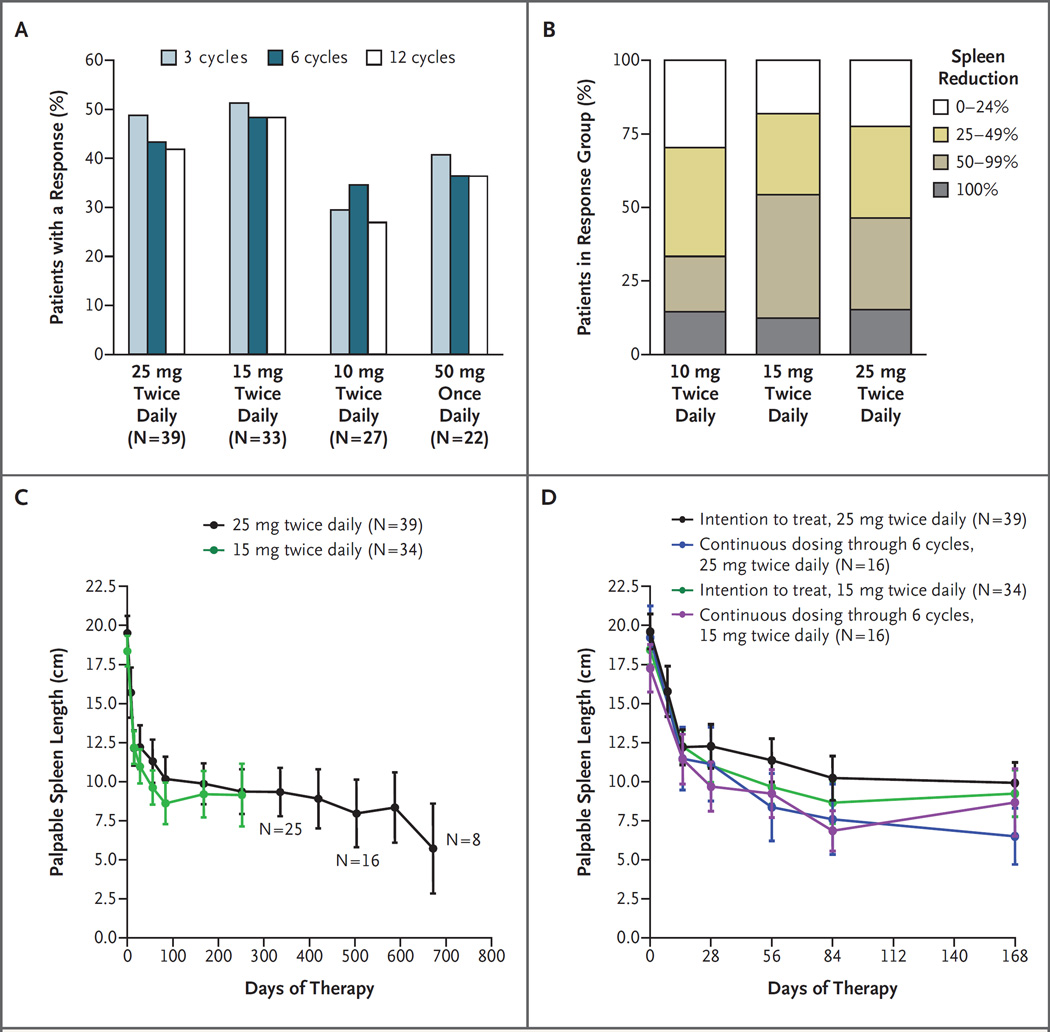
Figure 1. Clinical Responses to INCB018424 Therapy.
Patients who received INCB018424 had clinical improvement (according to the criteria of the International Working Group for Myelofibrosis Research and Treatment) on the basis of a reduction of 50% or more in palpable splenomegaly, which was durable for 12 cycles (12 months) (Panel A). The proportion of patients with a 0 to 24%, 25 to 49%, 50 to 99%, or 100% decrease in palpable spleen length relative to baseline was similar between the groups of patients in the intention-to-treat population who received 15 mg twice daily and 25 mg twice daily; 10 mg twice daily provided a lesser, but durable, response (Panel B). Reduction in the spleen size was rapid and durable for approximately 2 years, as shown by the mean spleen size over time. The initial response to INCB018424 therapy (reduction in mean spleen length) is shown for the patients who received 15 mg twice daily and 25 mg twice daily (Panel C) and for the subgroup of patients who received their originally assigned dose for at least 6 months (Panel D). I bars denote standard errors.