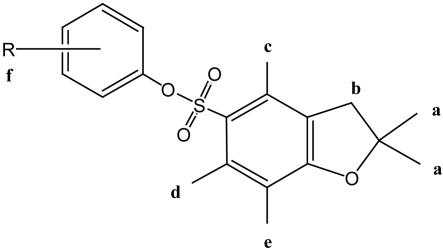
Table 1. Proton NMR data for PPBF and methylated analogs.
1H chemical shifts (ppm) for PPBF analogs were measured in deuterated chlorofom at 24.8°C. The spectrum of PPBF was characterized by the appearance of signals consistent with the 2,2,4,6,7-pentamethyl-dihydrobenzofuran nucleus linked to an isolated and otherwise unsubstituted phenyl ring. In particular, there was a resonance for the pair of 2,2 methyl groups (1.49 ppm; singlet; 6H), three additional resonances for the 4-, 6-, and 7-methyl groups (2.56, 2.33, 2.14 ppm; singlets; 3 × 3H) and a complex multiplet of aromatic signals (7.02-7.35 ppm) partially obscured by the residual CHCl3 resonance at 7.26 ppm, consistent with the isolated phenyl ring. NA, not applicable. R refers to the position at any methyl substitutions on the phenyl ring.