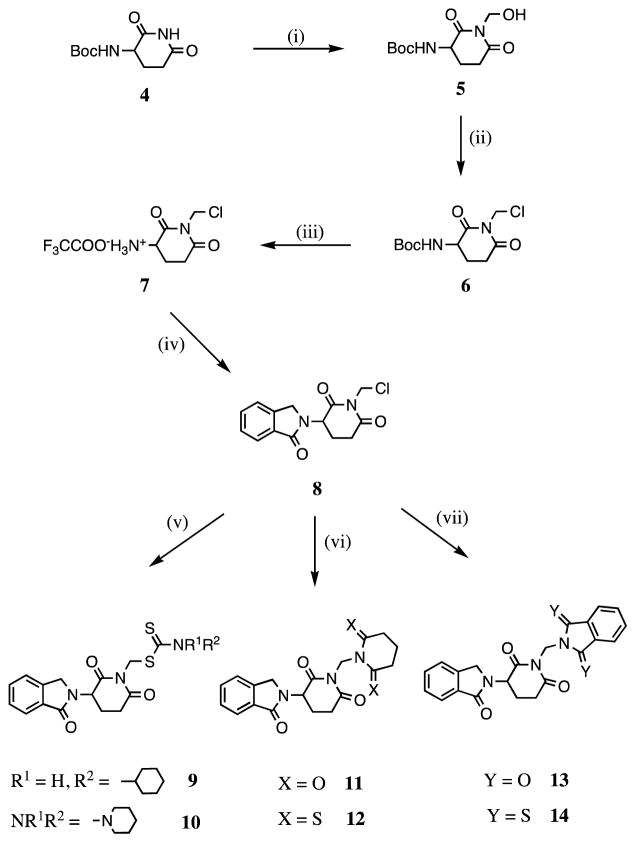
Scheme 1.
Reagents and conditions: (i) Formaldehyde (37% solution in water), N2, reflux, 0.5 h; (ii) thionyl chloride, DMF, 0 °C, 1 h; (iii) trifluoroacetic acid, CH2Cl2, N2, rt, 22.5 h; (iv) phthaldialdehyde, THF, N2, rt, 71 h; (v) carbon disulfide, cyclohexylamine (for 9), piperidine (for 10), CH3CN, N2, rt, 42 to 46 h; (vi) glutarimide (for 11), dithioglutarimide (for 12), KOH, CH3CN, N2, rt, 16 to 18 h; (vii) phthalimide (for 13), dithiophthalimide (for 14), KOH, CH3CN, N2,rt, 18 h.