Fig. 2.
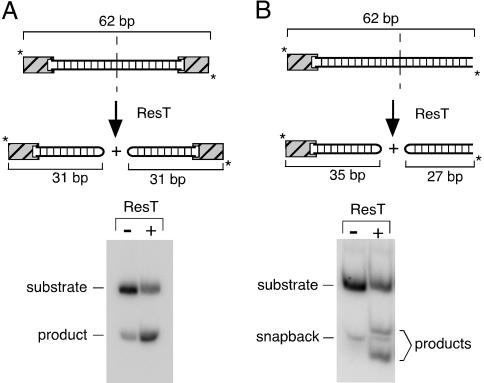
In vitro telomere resolution by ResT. (A Upper) Schematic of the telomere resolution reaction on a double-end-labeled (*), 62-bp, DNA-replicated telomere substrate. Addition of ResT leads to the production of 31-bp resolution products that contain a covalently closed hairpin end. The striped boxes at the ends of the substrate denote different GC clamps, which were required to maximize annealing of the two strands and to minimize snapback of the individual strands on themselves to form hairpins. (A Lower) Autoradiograph of an acrylamide gel showing bands corresponding to the unreacted substrate and the resolution products. A band running at the same position as the product is visible in the unreacted substrate because of some denaturation and subsequent snapback of the very A-T-rich substrate during the 30°C reaction incubation for 30 min. Controls without ResT were run for all substrates, and the background amount of 31-bp hairpin (typically ≈7%) was subtracted from ResT reaction signals. Reactions contained 2 nM labeled substrate and 80 nM ResT and were stopped by the addition of SDS. Products were analyzed on an 8% polyacrylamide gel (see Materials and Methods). (B Upper) Schematic of the telomere resolution reaction on a double-end-labeled (*), 62-bp, asymmetric, DNA-replicated telomere substrate. Addition of ResT leads to the production of 35- and 27-bp resolution products that migrate differently than the unreacted snapback DNA fragment. (B Lower) Autoradiograph of an acrylamide gel showing bands corresponding to the unreacted asymmetric substrate and resolution products.