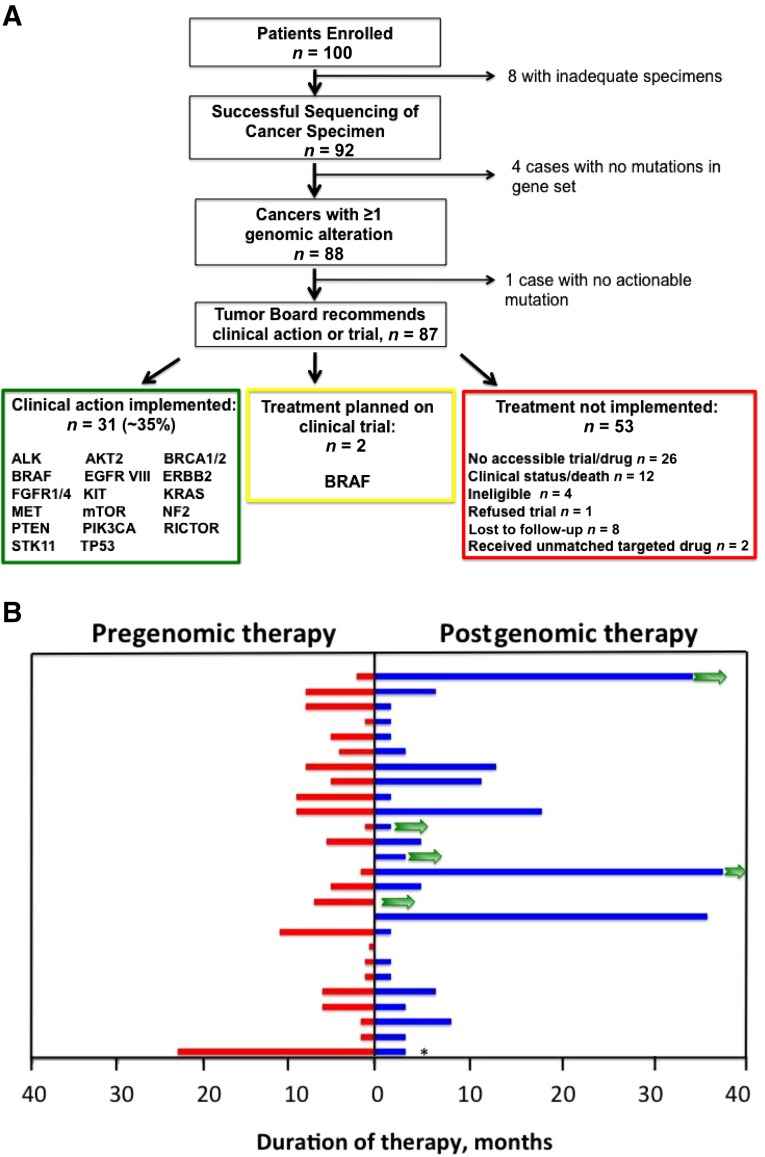
Figure 1.
Tumor genomic profiling consort diagram and patient outcomes. (A): Comprehensive genomic profiling of tumors from 100 patients with rare or refractory cancers. Time from genomic testing to generation of formal recommendations averaged 3–4 weeks. Clinical action included enrollment in a therapeutic clinical trial, FDA-approved therapy, off-label use of approved therapy, discontinuation of ineffective targeted therapy, germline mutation testing, and diagnostic reclassification. Genes with alterations for which clinical action was implemented are listed in the respective categories. Two patients are being screened for participation in clinical trial with targeted therapy. Two patients with action implemented had targeted therapy prescribed but were then lost to follow-up. (B): Duration of therapy in individual patients before genomic profiling and with targeted therapy (not significant). Comparisons are for nontargeted therapy before genomic profiling (red) and duration of targeted therapy after genomic profiling (blue). Green arrows indicate patients with ongoing therapy. Last patient was on combined therapy with targeted agent, which was discontinued (∗) at 5 months for side effects. Patient has ongoing response with monotherapy.
Abbreviation: FDA, U.S. Food and Drug Administration.