Table 2.
Substrate scope of Mb(L29S,H64V,V68F).[a]
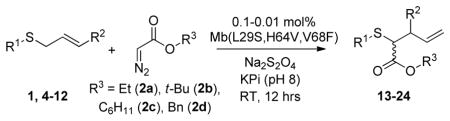
| |||||
|---|---|---|---|---|---|
| Sulfide | Diazo reag. | Product | % conv. (TON) | TTN[b] | ee [%][c] |
| 1 | 2b |
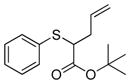 13 |
>99% (>1,000) | 8,170 | 6% |
| 1 | 2c |
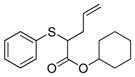 14 |
>99% (>1,000) | 8,820 | 9% |
| 1 | 2d |
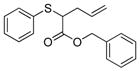 15 |
94% (940) | 3,570 | 47% |
| 4 | 2a |
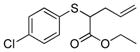 16 |
>99% (>1,000) | 5,050 | 20% −60%[d] |
| 5 | 2a |
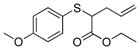 17 |
>99% (>1,000) | 5,960 | 40% |
| 6 | 2a |
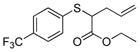 18 |
57% (570) | 1,000 | 18% 58%[d] |
| 7 | 2a |
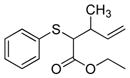 19 |
>99% (>1,000) | 8,120 | 57/59% 1:1 d.r. |
| 8 | 2a |
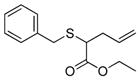 20 |
78% (780) | 7,040 | 10% |
| 9 | 2a |
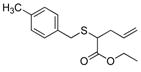 21 |
93% (930) | 5,470 | 43% |
| 10 | 2a |
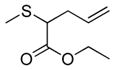 22 |
35% (350) | 3,570 | 38% |
| 11 | 2a |
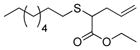 23 |
8% (80) | 125 | n.d.[e] |
| 12 | 2a |
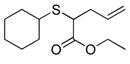 24 |
86% (860) | 4,190 | 19% |
Under standard reactions conditions as described in Table 1.
Using 1 μM Mb catalyst instead of 10 μM.
As determined by chiral GC or Supercritical Fluid Chromatography (SFC).
Using Mb(H64V,V68A).
Enantiomers could not be resolved.
