Abstract
Castrate-resistant prostate cancer (CRPC) progression is a complex process by which prostate cells acquire the ability to survive and proliferate in the absence or under very low levels of androgens. Most CRPC tumors continue to express the androgen receptor (AR) as well as androgen-responsive genes owing to reactivation of AR. Protein tyrosine kinases have been implicated in supporting AR activation under castrate conditions. Here we report that Lyn tyrosine kinase expression is upregulated in CRPC human specimens compared with hormone naive or normal tissue. Lyn overexpression enhanced AR transcriptional activity both in vitro and in vivo and accelerated CRPC. Reciprocally, specific targeting of Lyn resulted in a decrease of AR transcriptional activity in vitro and in vivo and prolonged time to castration. Mechanistically, we found that targeting Lyn kinase induces AR dissociation from the molecular chaperone Hsp90, leading to its ubiquitination and proteasomal degradation. This work indicates a novel mechanism of regulation of AR stability and transcriptional activity by Lyn and justifies further investigation of the Lyn tyrosine kinase as a therapeutic target for the treatment of CRPC.
Introduction
Prostate cancer (PCa) is the second leading cause of cancer-related death in North American men.1 Androgen-deprivation therapy has remained the standard first-line therapy for metastatic PCa for over 70 years, and newer treatments like abiraterone and enzalutamide continue to target the androgen receptor (AR) pathway.2,3 Although anti-androgen treatment causes initial tumor regression owing to apoptosis in androgen-sensitive tumor cells, most patients will suffer disease relapse and progress to castrate-resistant prostate cancer (CRPC) within 2–3 years of treatment initiation.4,5 CRPC progression is a complex process by which cells acquire the ability to survive and proliferate in the absence of androgens. The tumor develops mechanisms which reactivate the AR axis6 via oncogenic pathways, in which tyrosine kinases have a crucial role.7 For example, epidermal growth factor stimulation of PCa cells results in a rapid tyrosine phosphorylation of AR on Tyr267 and Tyr534 via the non-receptor tyrosine kinases, Ack1 and Src, which accelerates transcriptional activity of AR in androgen-deprived conditions.8,9 These findings revealed the important role of tyrosine phosphorylation in ligand-independent activation of AR and provided a new mechanism for activation of AR in the androgen-deprived environment.
The Lyn tyrosine kinase is a member of the src family of tyrosine kinases (SFKs), which regulate a variety of epithelial and hematopoietic cellular events, including cell differentiation, growth, proliferation, survival, cell adhesion and migration, as well as drug resistance.10, 11, 12 Current literature suggests that Lyn may have an important role in prostate development and cancer progression. For example, Lyn is expressed in normal prostate epithelium in humans13 and Lyn-deficient mice display compromised prostate gland development.14,15 In patients with PCa, increases in membrane-associated Lyn expression was associated with a shorter time to relapse and median time from diagnosis to hormone escape.13 Moreover, targeting Lyn expression with antisense, or Lyn activity using inhibitory peptides, decreases proliferation and tumor volume in PCa DU145 cells.16,17 These data suggest that Lyn expression and activity are important for PCa progression, however the underlying molecular mechanisms by which Lyn affects the proliferation and survival of prostate cells or tumors is yet to be defined.
In this study, we demonstrated that Lyn kinase expression correlates with progression from primary PCa to CRPC in human tumors and its overexpression accelerates CRPC progression in PCa xenografts in vivo. In vitro, Lyn is expressed more highly in androgen-independent PCa cell lines, and Lyn overexpression and silencing experiments showed that Lyn stabilizes AR expression by maintaining ARs association with the molecular chaperone Hsp90 and enhances AR transcriptional activity. Our results suggest, therefore, that specific targeting of Lyn kinase expression presents a potential therapeutic strategy for disrupting AR signaling in the castrated environment and may prevent CRPC development.
Results
Lyn expression is associated with progression to CRPC
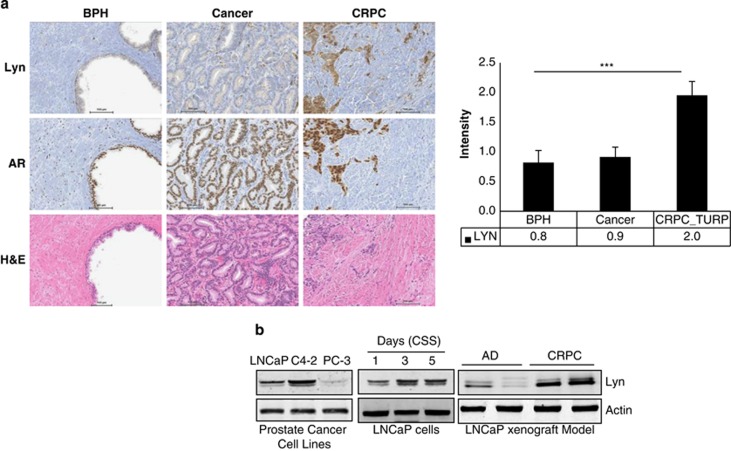
To elucidate the role of the Lyn tyrosine kinase in PCa progression to CRPC, immunohistochemistry analysis was performed using a human prostate tissue microarray. Lyn expression was analyzed in human specimens (normal prostate, primary tumors and CRPC) (Figure 1a and Supplementary Table S1). We found that Lyn expression did not change between normal and primary tumor, tissue, whereas Src was upregulated in primary cancer compared with normal tissue (Supplementary Figure S1 and Supplementary Table S2). These data suggest that Lyn is not involved in initial transformation to primary adenocarcinoma of the prostate and are in accordance with previously published data on human specimens and work showing that Lyn overexpression does not impact PCa development or increase susceptibility to tumors in transgenic mice.18 However, we did observe Lyn expression levels were on average twofold higher in CRPC specimens compared with primary hormone naive tumor specimens (Figure 1a). Next, we analyzed the expression of Lyn in three PCa cell lines routinely used in the field (LNCaP, C4-2 and PC-3), representing androgen-dependent and androgen-independent growth features, as well as positivity and negativity in terms of AR protein expression. We found that Lyn expression was lowest in AR-negative PC-3 cells compared with AR-positive LNCaP cells, and was expressed most highly in AR-positive, castrate-resistant C4-2 cells (Figure 1b, left). In order to test whether increased Lyn expression occurs in response to androgen deprivation, LNCaP cells were cultured in charcoal striped serum (CSS). Our results show that Lyn expression is upregulated in androgen-deprived conditions in a time-dependent manner, which is concordant with recently published data19 (Figure 1b, middle). Similar results were also observed in vivo using an LNCaP CRPC xenograft model. In this system, LNCaP PCa cells were grown subcutaneously in vivo in non-castrated male nude mice (androgen-dependent phase). After serum levels of prostate specific antigen (PSA) reached 50 ng/ml or above, mice were castrated and tumors regressed prior to regrowing in an androgen independent, or castration resistant, phase of disease. We found that Lyn expression was increased in CRPC LNCaP tumors compared with tumors that grew during the androgen-dependent phase of disease (Figure 1b, right). These findings suggest that Lyn expression in PCa cells is regulated by androgen deprivation and correlates with progression to CRPC in vitro, in vivo, and in human specimens.
Figure 1.
Lyn expression is associated with progression to CRPC. (a) Lyn expression is upregulated in human CRPC. Lyn and AR expression were evaluated in human tissue specimens by immunohistochemistry from patients with benign prostatic hyperplasia, primary hormone naive PCa, as well as CRPC (TURP-transurethral resection of prostate) (***P<0.0001), lower panel shows H&E staining. (b) Lyn expression is upregulated in androgen-independent cell lines and xenografts: proteins were extracted from LNCaP, C4-2 and PC-3 cells (left panel), LNCaP cells cultured in CSS over time (middle panel), and LNCaP xenografts before castration (AD) or after progression to CRPC (right panel). Western blots were performed using Lyn antibody and actin was used as loading control.
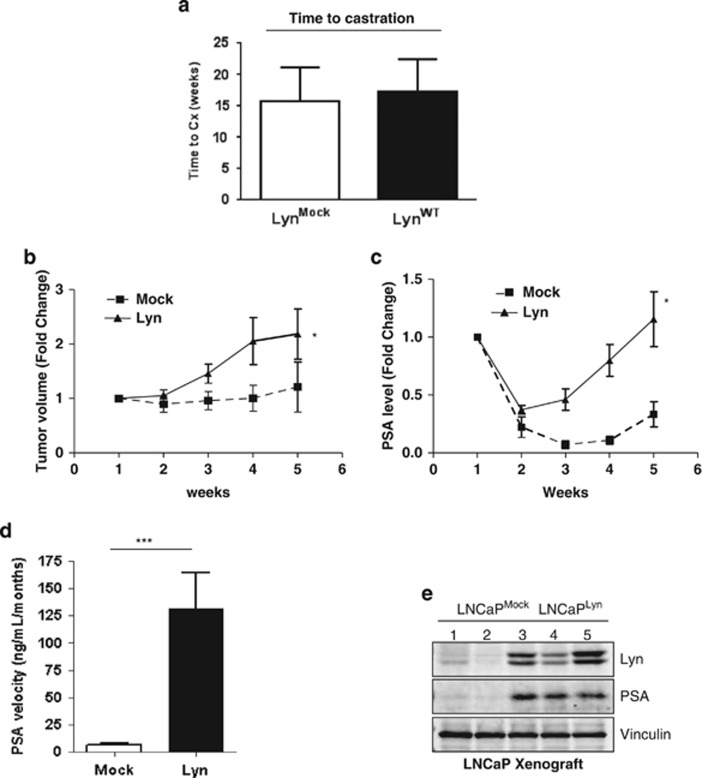
To determine whether Lyn expression may drive CRPC progression, LNCaP cells were stably transfected with Lyn (LNCaPLyn) or empty vector (LNCaPMock) and their capacity to modulate CRPC in vivo was tested. No difference was observed between growth of tumors harboring Lyn or empty vector before castration (Figure 2a). After castration, however, we observed that mice bearing LNCaPLyn tumors showed increase of tumor volume (Figure 2b) and a rapid increase of serum PSA (Figure 2c) and PSA velocity (Figure 2d) compared with mice bearing LNCaPMock tumors. Moreover, we found that PSA expression at protein levels was higher in LNCaPLyn tumors compared with the LNCaPMock tumors (Figure 2e) further supporting our data from serum circulating PSA. Together, these data indicate that Lyn overexpression accelerates the time to CRPC progression, with increased activation of the AR axis in vivo.
Figure 2.
Lyn overexpression accelerates progression to CRPC in vivo. (a) Lyn overexpression increases tumor volume post castration. LNCaPLyn and LNCaPMock cells were injected into male athymic nude mice, followed by castration when serum PSA reached 50 ng/ml. Mean growth time before castration was evaluated in weeks. (b) Lyn overexpression increases tumor volume. Mean tumor volume of LNCaPLyn and LNCaPMock xenografts tumors post castration over time (*P<0.05; data represent average±s.e.m. of six mice/group). (c) Lyn overexpression increases serum PSA post castration. Serum PSA levels post castration over time (*P<0.05; data represent average±s.e.m. of six mice/group). (d) Lyn overexpression increases PSA velocity post castration. PSA velocity was calculated from the time of castration to the time of killing (***P<0.0001; data represent average±s.e.m. of six mice/group). (e) Lyn overexpression increases PSA levels in LNCaP xenografts. Total protein was extracted from the LNCaPLyn and LNCaPMock xenograft tumors and western blot was performed using Lyn and PSA antibodies, Vinculin antibody was used as a loading control.
Lyn regulates AR transcriptional activity
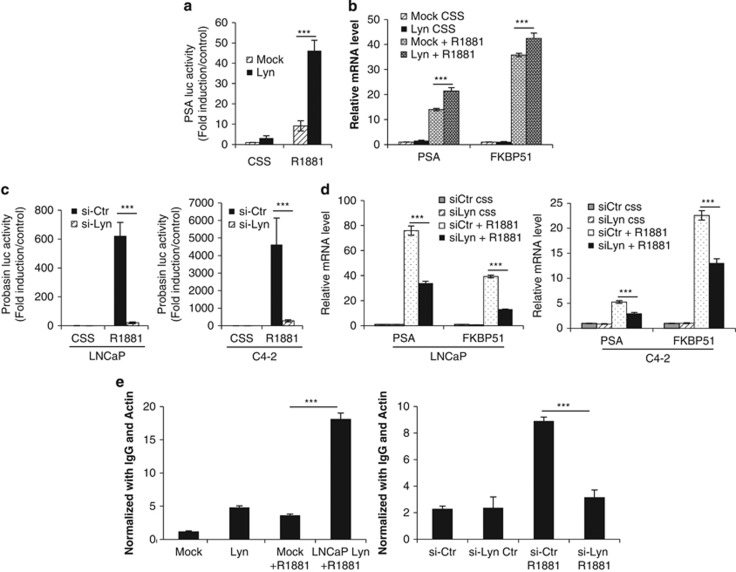
Based on these results showing increased PSA levels with Lyn overexpression in vivo, we hypothesized that Lyn enhances AR transcriptional activity. PSA transcriptional activity was analyzed after gain and loss of Lyn expression using a PSA or Probasin luciferase reporter assay as well as mRNA expression of PSA and the AR downstream gene FKBP51. LNCaPLyn cells exhibited increased AR transcriptional activity by approximately sixfold compared LNCaPMock at basal levels, and this effect was amplified in the presence of the synthetic androgen R1881 by up to 40-fold (Figure 3a). This effect was also observed using two clones with different Lyn expression levels (Supplementary Figure S2) At the mRNA level, LNCaPLyn cells expressed higher levels of PSA and FKBP51 after R1881 stimulation (Figure 3b). Reciprocally, our results in LNCaP and C4-2 cells treated with Lyn siRNA showed that Lyn is required for increased AR transcriptional activity in the presence of R1881 (Figure 3c) as well as expression of AR-dependent genes (Figure 3d). Similar effect of Lyn knockdown on AR transcriptional activity using two clones with different Lyn shRNA sequence was also observed (Supplementary Figure S3). Accordingly, we found that Lyn overexpression induced increase AR binding of AR to the promoter proximal region (ARE I) of PSA by fivefolds at basal level, which was enhanced by R1881 (Figure 3e, left), whereas Lyn knockdown completely abrogated R1881-induced AR binding (Figure 3e, right). Taken together, these results indicate that Lyn is a potent inducer of AR transcriptional activity that is associated with progression to CRPC.
Figure 3.
Lyn regulates AR transcriptional activity. (a) Lyn overexpression increases AR transcription. LNCaPLyn and LNCaPMock cells were transfected with PSA luciferase and were treated ±R1881 for 24 h and PSA luciferase activity was determined. Data represent mean±s.e.m. of at least three independent experiments done in triplicate(left panel). The results were reported as mean±s.d.; ***P<0.0001. (b) Lyn overexpression increases AR downstream genes. LNCaPLyn and LNCaPMock were cultured in CSS for 24 h prior to stimulation ±R1881 for 24 h. RNA was extracted and qRT-PCR was performed to evaluate the expression levels of PSA and FKBP51, which were normalized by GAPDH (right panel). The results were reported as mean±s.d.; ***P<0.0001. (c) Lyn knockdown abrogates R1881-induced AR transcriptional activity. LNCaP and C4-2 cells transfected with 10 nM Lyn or Ctrl siRNA along with PSA or Probasin luciferase and were treated ±R1881 for 24 h PSA and Probasin luciferase activity were determined. Data represent mean±s.e.m. of at least three independent experiments done in triplicate. The results were reported as mean±s.d.; ***P<0.0001. (d) Lyn knockdown abrogates R1881-induced AR downstream genes. LNCaP and C4-2 cells transfected with 10 nM Lyn or Ctr siRNA alone were cultured in CSS for 24 h prior to stimulation ±R1881 for 24 h. RNA was extracted and qRT-PCR was performed to evaluate the expression levels of PSA and FKBP51, which were normalized by GAPDH (right panel). The results were reported as mean±s.d.; ***P<0.0001. (e) Lyn expression regulates AR recruitment to the PSA promoter. LNCaPLyn and LNCaPMock (left panel) or LNCaP cells treated with 10 nM siRNA-Lyn or siRNA-Ctr (right panel) were treated ±R1881 for 24 h, chromatin immunoprecipitation was performed using AR antibody, along with IgG as control and qRT-PCR was performed using the primers for ARE I. The results were reported as mean±s.d.; ***P<0.0001.
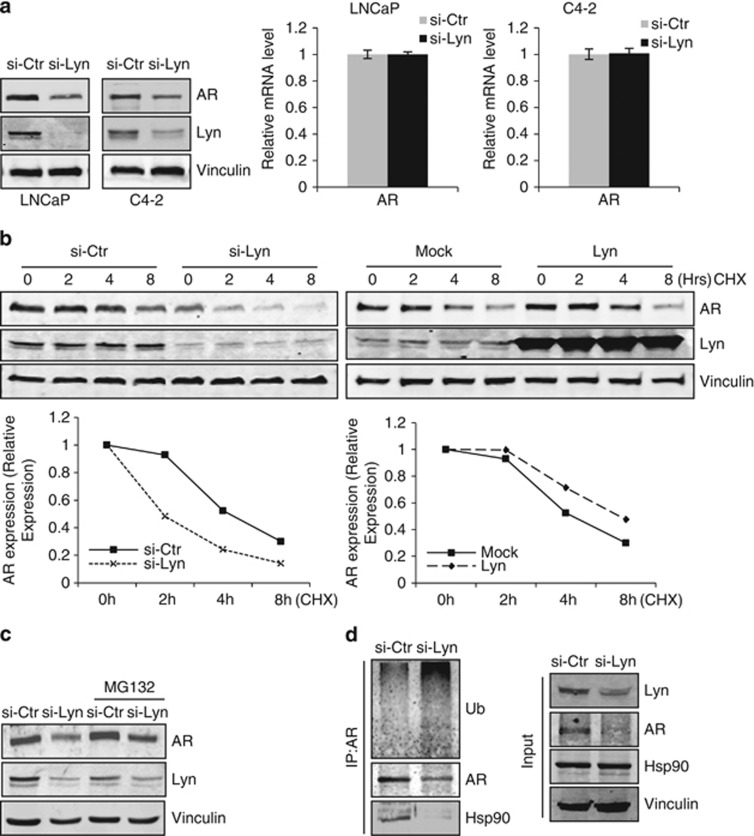
Lyn stabilizes AR expression at the protein level
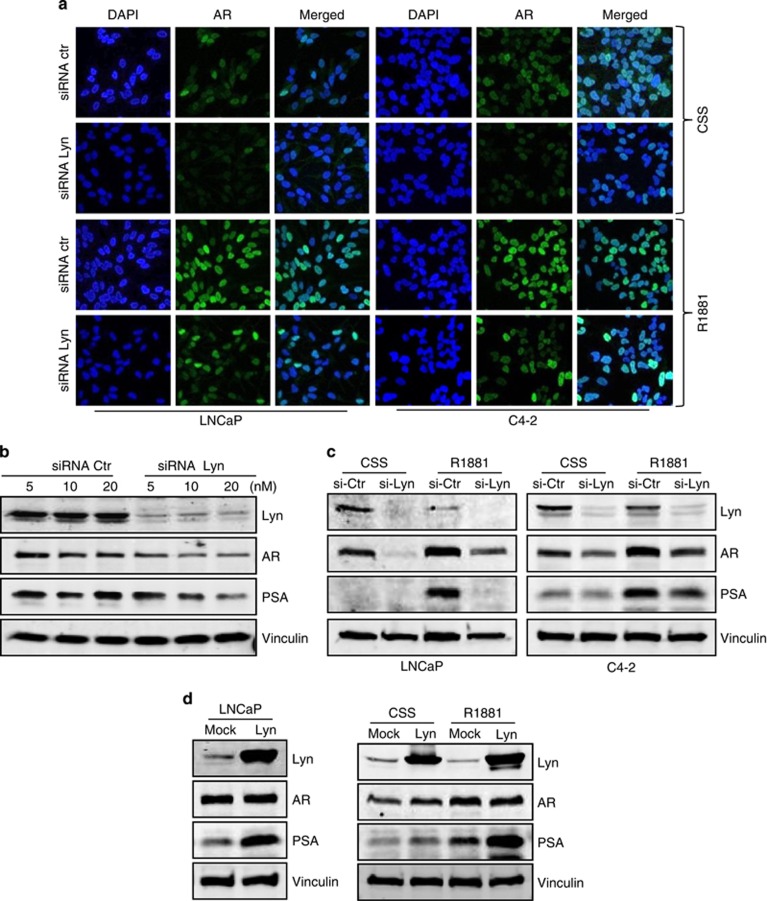
To initiate transcription of downstream genes, AR must translocate to the nucleus. Thus, to investigate the mechanism by which Lyn regulates AR transcriptional activity, we assessed if Lyn is required for AR expression or its nuclear translocation. Using immunofluorescence, we observed that Lyn knockdown by siRNA in both LNCaP and C4-2 cells decreased the intensity of AR staining compared with control in CSS conditions (Figure 4a, top rows). Although Lyn knockdown did not prevent R1881-induced AR nuclear translocation, we noticed a similar reduction in AR staining intensity after R1881 treatment (Figure 4a, bottom rows), suggesting that it is the effect of Lyn knockdown on AR expression rather than nuclear translocation that leads to decreased transcriptional activity observed in these cells (Figures 3c and d). Indeed, our results support this hypothesis, as we found that Lyn siRNA induced a profound decrease of AR and PSA as well as Lyn in a dose-dependent manner (Figure 4b) and this was also observed using different sequence of Lyn siRNA (Supplementary Figure S4). This effect on AR expression was not seen after Src knockdown (Supplementary Figure S5). This suppression of AR expression correlated with a decrease of PSA even in the presence of R1881 in both LNCaP and C4-2 cells (Figure 4c). Reciprocally, overexpressing LNCaPLyn cells showed increased AR expression in fetal bovine serum (Figure 4d, left), CSS and CSS+R1881 conditions (Figure 4d, right). Together these data suggest that Lyn regulates AR expression at the protein level.
Figure 4.
Lyn stabilizes AR protein expression. (a) Lyn knockdown reduces AR expression and nuclear translocation. LNCaP cells transfected with 10 nM Lyn or Ctr siRNA in the presence of CSS for 24 h and were treated ±R1881. Immunofluorescence was performed using AR (green) and DAPI (nuclei-blue). (b) Lyn knockdown decreases AR and PSA protein expression. LNCaP were treated with Lyn or Ctr siRNA is a dose-dependent manner. Total proteins were extracted and western blots were performed using Lyn, PSA, AR antibodies and vinculin was used as a loading control. (c) Lyn knockdown abrogates R1881-induced PSA at protein levels. LNCaP and C4-2 were transfected with 10 nM of Lyn and Ctr siRNA and were treated ±R1881. Total proteins were extracted and western blots were performed using Lyn, PSA, AR and vinculin was used as a loading control. (d) Lyn overexpression increases AR and PSA protein expression. Total proteins were extracted from LNCaPLyn and LNCaPMock cultured in FBS (left panel) or from LNCaPLyn and LNCaPMock cultured for 24 h in CSS and treated ±R1881 (right panel). Western blots were performed using Lyn, PSA, AR antibodies and vinculin was used as a loading control.
Lyn prevents proteasomeal degradation of the AR
The requirement for Lyn in maintaining AR expression using siRNA knockdown of Lyn was observed at the protein (Figure 5a, left) but not mRNA levels in LNCaP and C4-2 cells (Figure 5a, right). Therefore, we investigated the effects of Lyn knockdown and overexpression on AR protein stability over time using cycloheximide-treated LNCaP cells. As shown in Figure 5b (left), AR protein levels decreased significantly in Lyn siRNA treated cells after 2 h compared with control, which maintained discernable levels of AR for up to 8 h post cycloheximide treatment. Reciprocally, Lyn overexpression prolonged AR half-life compared with Empty vector-transfected controls (Figure 5b, right).
Figure 5.
Lyn knockdown induces AR degradation via the proteasome. (a) Lyn knockdown decreases AR expression at the protein but not mRNA level. LNCaP and C4-2 cells were treated with 10 nM of Lyn or control siRNA. Total proteins were extracted and western blots were performed using Lyn and AR antibodies, Vinculin antibody was used as a loading control (left panel). RNAs were extracted and qRT-PCR was performed to evaluate the expression level of AR, which was normalized by GAPDH (right panel). (b) Lyn expression stabilizes AR. LNCaP cells transfected with 10 nM Lyn or control siRNA (left panel) and stably transfected cells LNCaPLyn and LNCaPMock (right panel) were treated with 10 μM cycloheximide for indicated time period. Total proteins were extracted and western blots were performed using AR and Lyn antibodies, Vinculin was used as a loading control. Intensity of AR was quantified using ImageJ software and normalized to vinculin expression. (c) Lyn expression protects AR from proteasomal degradation. LNCaP cells were transfected with 10 nM Lyn or control siRNA and were treated with 10 uM MG-132 for 6 h. Total proteins were extracted and western blots were performed using Lyn and AR antibodies, vinculin was used as a loading control. (d) Lyn knockdown induces dissociation of AR from Hsp90 and increases AR ubiquitination. LNCaP cells were transfected with 10 nM Lyn or control siRNA, and immunoprecipitation of AR was performed followed by western blot using ubiquitin, Hsp90 and AR antibodies (left panel). Input was blotted with AR, Lyn and Hsp90 antibodies and Vinculin antibody was used as a loading control (right panel).
Based on our previous results showing the requirement for Lyn in maintaining AR protein levels and as AR can be degraded through the proteasome,20,21 we then tested whether Lyn protects AR from proteosomal degradation. To do so, siLyn and siCtr LNCaP cells were treated with the proteasome inhibitor MG-132. Our data revealed that MG-132 abrogated Lyn knockdown-induced decrease of AR expression, suggesting that in the absence of Lyn there is enhanced degradation of AR via the proteasome (Figure 5c). To investigate the molecular mechanism by which Lyn protects AR from proteasomeal degradation, we questioned whether Lyn was required for the interaction between AR and the molecular chaperone Hsp90, which provides stability for ligand-unbound AR.22,23 Our data indicated that targeting Lyn kinase by siRNA disrupted the association between AR and Hsp90 and enhanced AR ubiquitination (Figure 5d). These data suggest that Lyn knockdown results in dissociation of AR from Hsp90, which in turn leads to ubiquitination of unstably-folded AR and its degradation by the proteasome. Taken together, our results show that Lyn not only regulates AR transcriptional activity and nuclear translocation, but it is also required for AR stability at the protein level.
Lyn is required for progression to CRPC in vivo
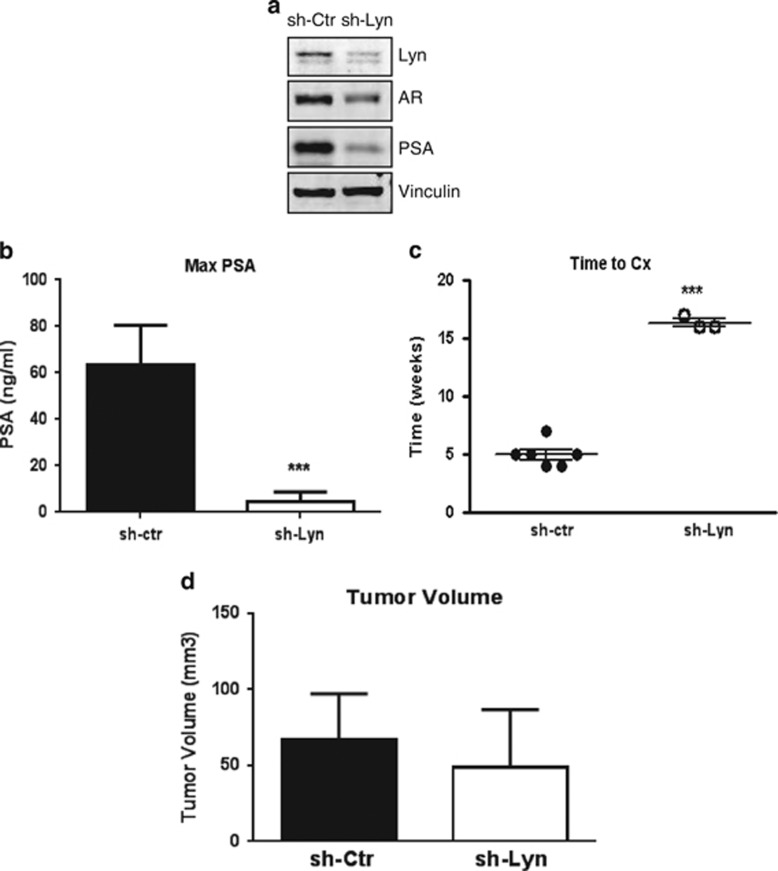
Our initial observations in human tumors and in mice bearing Lyn overexpressing xenografts suggested that Lyn has a role in the progression to CRPC. Moreover, we found that Lyn was required for stabilizing AR protein levels, thereby regulating AR transcriptional activity. As AR activation is a hallmark of CRPC, we finally investigated the effects of Lyn knockdown in our in vivo CRPC xenograft model. Our data showed that as with siRNA treatment, stable Lyn knockdown in LNCaP cells using shRNA (LNCaPsh-Lyn) resulted in decreased expression of the AR and PSA (Figure 6a) compared with sh-Ctr (LNCaPsh-Ctr) cells, further confirming the role of Lyn in AR signaling pathway. As Lyn is upregulated in CRPC patient specimens and Lyn is required for AR stability, we investigated if Lyn knockdown will influence progression to CRPC. We found that that serum PSA was significantly reduced in mice bearing LNCaPsh-Lyn xenografts compared with LNCaPsh-Ctr xenografts (Figure 6b). This resulted in significant increased time to castration in mice with LNCaPsh-Lyn tumors, with only three out of eight reaching the PSA cutoff for castration by 16 weeks compared with LNCaPsh-Ctr (Figure 6c). As a result, time to CRPC could not be accurately assessed in LNCaPsh-Lyn tumors compared with LNCaPsh-Ctr, underscoring the requirement for Lyn in CRPC. Importantly, however, Lyn silencing did not affect cell proliferation in vitro (Supplementary Figure S6) or tumor volume prior to castration and found that LNCaPsh-Lyn xenografts grew at the same rate as LNCaPsh-Ctr xenografts before castration (Figure 6d). Together with our in vivo data from mice bearing Lyn overexpressing tumors, these results suggest that Lyn expression is important for activation of the AR-mediated progression in CRPC, by maintaining AR stability and subsequent transcriptional activity.
Figure 6.
Lyn is required for CRPC progression in vivo. (a) Stable Lyn knockdown reduces AR expression in vitro. Total proteins from LNCaPsh-Lyn and LNCaPsh-Ctr cells were extracted and western blot was performed using AR, PSA and Lyn antibodies and Vinculin was used as a loading control. (b) Lyn knockdown decreases PSA: maximum of PSA was measured in mice bearing LNCaPsh-Ctr tumors prior to castration and in mice bearing LNCaPsh-Lyn at 7 weeks (***P<0.0001). (c) Lyn knockdown prolongs time to castration: average time post inoculation to reach serum PSA of 50 ng/ml required for castration represent eight mice/group ±s.e.m. (***P<0.0001). (d) Lyn knockdown does not affect tumor volume pre castration, tumor volume was measured prior to castration. Data represent five mice/group ±s.e.m.
Discussion
In androgen-dependent tumors, prostate cells proliferate as a result of androgen stimulation.24 By contrast, during the progression of CRPC, prostate cells increase their sensitivity to the low levels of androgens and reactivation of the AR occurs by a variety of mechanisms, including hyperactivation of oncogenic signaling pathways. As AR activity is a known driver of CRPC,25,26 it is essential to identify targeted therapies that may allow AR activation under low androgen conditions. In this work, we have shown that the Lyn tyrosine kinase may be a viable target to prevent AR activity in CRPC. As targeting SFKs is being actively explored for multiple solid tumors, including prostate,27 our work suggests the utility of Lyn specific therapies in the prevention or treatment of CRPC.
The association of various SFKs with the development of PCa has been shown by different groups and recent reports suggest a distinct role for specific SFKs in the initiation of primary PCa or progression of disease to CRPC. For example, unlike Lyn, Src and Fyn have been reported to have tumorigenic capacity and have roles in PCa disease initiation.18 Our immuohistochemistry analysis of human tissue specimens demonstrated that whereas expression of Lyn kinase increased more than twofold in CRPC tissue samples compared with PCa hormone naive tumors, its expression did not change from normal to cancer tissue specimens (Figure 1a). By contrast, whereas Src kinase expression did not significantly increase in CRPC tissue specimens compared with PCa hormone naive tumors, Src expression did significantly increase in cancerous tissue compared with normal prostate tissue (Supplementary Figure S1). Supporting the hypothesis that Lyn has an important role in the progression of CRPC but not primary cancer (where sensitivity to low androgen conditions is not required), we also demonstrated that stable overexpression of Lyn was sufficient to accelerate progression to CRPC, but not tumor growth or PSA production in the androgen-dependent phase of growth in an LNCaP xenograft model (Figure 2). Furthermore, we observed an increase in Lyn expression levels in androgen-independent vs dependent PCa cell lines, especially after androgen withdrawal (Figure 1b), further suggesting that Lyn is upregulated by androgen deprivation.
Investigations into the mechanism by which CRPC is accelerated by the overexpression of Lyn indicated that Lyn expression directly correlates with AR activity and expression. This was first observed in vivo, where Lyn overexpressing tumors produced significant amounts of PSA in serum and intratumorally compared with control (Figure 2), which was associated with rapid tumor growth in the CRPC phase. In vitro experiments using Lyn overexpressing and siRNA transfected cells confirmed that Lyn expression was required for AR transactivation of downstream genes (Figure 3). This effect on AR transcriptional activity was due to the ability of Lyn to stabilize AR protein by facilitating its interaction with Hsp90, preventing its ubiquitnation and degradation by the proteasome (Figure 5). Finally, we reiterated the importance for Lyn in regulating AR expression and activity, which is required for the development of CRPC In our in vivo experiment, we showed that mice bearing stable Lyn knockdown tumors did not reach serum PSA levels required for castration (Figure 6).
To our knowledge, Lyn is the only known tyrosine kinase that affects the stability of AR at protein levels, whereas other kinases like Src and Ack1, have been reported to regulate AR transcriptional activity via phosphorylation.8,9 For example, Ack1 and Src phosphorylate AR, unraveling the molecular basis for interplay between Ack1/AR and Src/AR signaling in the progression of PCa.8,9 Activated Ack1 phosphorylates AR on Tyr267 and Tyr363 9 whereas Src phosphorylates AR on Tyr534.8,28 In conditions permissive to Src- or Ack-mediated phosphorylation, the stability of AR may not be an issue because of constant presence of ligand or stimulation of other oncogenic pathways, which are known activators of the AR. However, our results suggest that in castrate conditions, lack of ligand requires increased stability of unbound AR, which may be facilitated by Lyn via Hsp90 allowing for its increased activity. This hypothesis is supported by our data showing that overexpression of Lyn is sufficient to increase AR transcriptional activity even in CSS conditions alone (Figure 3). Moreover, there is precedent in the literature for association between Lyn and Hsp90. For example, in B-cell chronic lymphocytic leukemia cells, Lyn is tightly associated with Hsp90 and cell death after treatment with an Hsp90 inhibitor is associated with dissociation of Lyn from this complex.29 In addition, the importance of Lyn as a scaffolding protein has been shown in transgenic kinase-dead mutant mice, which have severe perturbations in immune function.30,31 In addition to protein stabilization, however, Lyn indeed may also have a role in AR phosphorylation. Thus, Lyn may support sustained AR activity in castrate conditions in PCa through an amplification loop of stabilization combined with increased phosphorylation, a possibility that we are actively investigating.
Collectively, our results demonstrate that Lyn is a critical regulator of AR expression and activity, particularly in androgen-deprived conditions. These data strongly support the importance of Lyn in the continued activation of the AR under low-ligand conditions found in CRPC. Importantly, our results underscoring the importance of Lyn, but not Src, in the progression of CRPC suggest a possible mechanism as to why the SFK inhibitor, Dasatinib, failed in clinical trials in CRPC patients.32 Dasatinib is a notoriously promiscuous inhibitor of SFKs as well as non-kinase targets33 that has a three times greater specificity for Src than Lyn.34 This suggests that as our data would predict, targeting Src is ineffective in CRPC patients and that Lyn may not have been a therapeutic target in these failed trials. Thus, our data support ongoing trials using a more Lyn specific inhibitor, such as Bafetinib,35 which binds Lyn with 100 times greater specificity than to Src,36 and suggest that such investigations (NCT01215799) may show even more efficacious results than those using broad SFK targeting agents, particularly if sequenced in early onset CRPC.
Materials and methods
Plasmid, reagents and antibodies
Lyn wild-type and empty vector plasmids were generously provided by Dr Yamaguchi (Department of Molecular Cell Biology, Chiba University, Chiba, Japan).37 siRNA and shRNA plasmids were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for AR (N-20), Lyn (H-6), PSA (C-19), Ubiquitin, Hsp90 and Actin were from Santa Cruz Biotechnology. Antibody for Vinculin was from Sigma Aldrich (Oakville, ON, Canada). Transfection reagents including Lipofectin, Oligofectamine, and OPTI-MEM media were from Life Technologies, Inc., Burlington, ON, Canada. Cell culture reagents including RPMI 1640, fetal bovine serum, CSS were from Life Technologies. R1881 was from PerkinElmer (Woodbridge, ON, Canada). Cycloheximide and MG-132 were from Calbiochem (Billerica, MA, USA).
Cell culture and transfection
LNCaP and C4-2 cells were kindly provided by Dr Leland W.K. Chung (1992, MDACC, Houston, TX, USA) and tested and authenticated by whole-genome and whole-transcriptome sequencing on Illumina Genome Analyzer IIx platform in July 2013. Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum. Cells were cultured in a humidified 5% CO2/air atmosphere at 37 °C. LNCaP cells were stably transfected with Lyn or empty vector control, sh-Lyn or sh-Control as previously described.38 For siRNA, cells were transiently transfected with 10 nM of siRNA-Lyn or control using oligofectamine transfection reagents in OPTI-MEM media as previously described.39 For siRNA experiments, cells were changed to media containing 10% fetal bovine serum for 48 h prior to harvest or further experimentation.
Immunohistochemistry
PCa tissue specimens were obtained from Vancouver Prostate Centre Tissue Bank. The H&E slides were reviewed to mark the desired areas and the corresponding paraffin blocks. Three tissue microarrays were manually constructed (Beecher Instruments, Sun Prairie, WI, USA) by punching duplicate cores of 1 mm for each sample. All specimens were from radical prostatectomy except 20 CRPC samples, which were obtained from transurethral resection of the prostate. Immunohistochemical staining was conducted by Ventana autostainer model Discover XT (Ventana Medical System, Tuscan, AZ, USA) with enzyme-labeled biotin streptavidin system and solvent-resistant DAB Map kit using 1:50 dilution of AR (N-20) and 1:10 dilution of Lyn (H-6), and Src antibodies. Specimens were graded from 0 to +3 intensity representing no staining to heavy staining by visual scoring. Automated quantitative image analysis was conducted using pro-plusimage software.
Western blot analysis and immunoprecipitation
Total proteins were extracted using lysis buffer and immunoprecipitation using 2 μg of AR followed by western blot as previously reported.20,39
Quantitative PCR
Total RNA was e0xtracted from cells using TRIzol reagent (Life technology). Total RNA (2 μg) was reversed transcribed using MMLV reverse transcriptase and random hexamers (Life technology) as previously reported.38 Real time monitoring of PCR amplification of cDNA was performed using the following primer pairs and probes Lyn (Hs00176719_m1), AR (Hs00171172_m1), PSA (Hs00426859_g1), FKBP51 (Hs01561006_m1), and GAPDH (Hs03929097_g1) on ABI ViiA 7 Real Time PCR System with TaqMan Gene Expression Master Mix (Applied Biosystems, Burlington, ON, Canada). Target gene expression was normalized to GAPDH levels in respective samples as an internal control. The results represent the quantification of gene expression from three independent experiments with each sample ran in triplicate.
Immunofluorescence
LNCaP and C4-2 cells were transfected with Lyn siRNA or control siRNA and were treated ±R1881 for 6 h. Cells were fixed and stained with AR as previously reported.20 Antigen was visualized using anti-rabbit antibody coupled to FITC (fluorescein isothiocyanate). Nuclei were visualized by DAPI fluorescence incorporated in the mounting media (Vectashield, Vector Laboratories, Burlingame, CA, USA). Photomicrographs were taken at × 40 magnification using Zeiss LSM 780 confocal microscope. Results are representative of random pictures taken from three independent experiments.
Luciferase assay
For luciferase reporter assays, PSA luciferase or Probasin luciferase plasmids were transfected as above together with the addition of a control Renilla plasmid (normalized to 2 μg/well) as we previously described.20 24 h post transfection, media was replaced by CSS ±R1881 for 24 h. Luciferase activities measured using the microplate luminometer (EG&G Berthold, Bad Wildbad, Germany). All experiments were carried out in triplicate.
Chromatin immunoprecipitation
LNCaP cells were plated and treated with Lyn siRNA or scrambled siRNA control, or transfected with Lyn wild-type or Empty vector as described above and were treated with 10 nM of R1881 for 4 h. Cells were then cross-linked with paraformaldehyde and sonicated. Chromatin immunoprecipitation assay was performed using EZ ChIP kit according to the manufacture (Upstate, Lake Placid, NY, USA) on the PSA gene regions ARE I as we previously described.20
Protein stability and proteasome inhibitor assays
LNCaP cells were plated and treated with Lyn siRNA or scrambled siRNA control, or transfected with Lyn wild-type or Empty vector as described above. For protein stability, media was changed 48 h later to RPMI+5% serum containing 10 μM of cycloheximide incubated at 37 °C for 2, 4, or 8 h as previously described.20 For proteasome inhibitor assays, siRNA treated cells were treated with 10 uM MG-132 for 6 h. Total protein was harvested for western blot using AR, Lyn and Vinculin antibodies.
In vivo experiments
Male athymic nude mice (Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA) were injected subcutaneously with 1 × 106 LNCaPLyn, LNCaPMock, LNCaPsh-Lyn or LNCaPsh-ctrl cells. When tumors reached 300 to 500 mm3 or when serum PSA was >50 ng/ml, mice were castrated. Tumor volume and serum PSA were measured as previously described.40 All animal procedures were performed according to the guidelines of the Canadian Council on Animal Care.
Statistical analysis
All data were analyzed by two-tailed unpaired student's t-test or one-way analysis of variance with post hoc test. Overall survival was analyzed using Kaplan–Meier curves, and statistical significance between the groups was assessed with the log-rank test (GraphPad Prism). The results were reported as mean±s.d.; and statistical significance were set at ***P<0.0001 and *<0.05.
Acknowledgments
This work was supported by Terry Fox Research Institute new investigator grant (F10-03364) & Michael Smith Foundation for Health Research (A. Zoubeidi).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis).
Supplementary Material
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- Ryan CJ. Abiraterone: current and future use in prostate cancer. Clin Adv Hematol Oncol 2012; 10: 180–181. [PubMed] [Google Scholar]
- Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol 2001; 166: 500–506. [PubMed] [Google Scholar]
- Gleave M, Goldenberg SL, Bruchovsky N, Rennie P. Intermittent androgen suppression for prostate cancer: rationale and clinical experience. Prostate Cancer Prostatic Dis 1998; 1: 289–296. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res 2009; 15: 4792–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci USA 2012; 109: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell 2006; 10: 309–319. [DOI] [PubMed] [Google Scholar]
- Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci USA 2007; 104: 8438–8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benati D, Baldari CT. SRC family kinases as potential therapeutic targets for malignancies and immunological disorders. Curr Med Chem 2008; 15: 1154–1165. [DOI] [PubMed] [Google Scholar]
- Engen JR, Wales TE, Hochrein JM, Meyn MA 3rd, Banu Ozkan S, Bahar I et al. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci 2008; 65: 3058–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 2003; 101: 690–698. [DOI] [PubMed] [Google Scholar]
- Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res 2009; 15: 3540–3549. [DOI] [PubMed] [Google Scholar]
- Goldenberg-Furmanov M, Stein I, Pikarsky E, Rubin H, Kasem S, Wygoda M et al. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res 2004; 64: 1058–1066. [DOI] [PubMed] [Google Scholar]
- Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res 1994; 54: 2577–2581. [PubMed] [Google Scholar]
- Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res 2008; 68: 3323–3333. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 1991; 64: 693–702. [DOI] [PubMed] [Google Scholar]
- Cai H, Smith DA, Memarzadeh S, Lowell CA, Cooper JA, Witte ON. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci USA 2011; 108: 6579–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan P, Dalgliesh C, Carling PJ, Buist T, Zhang C, Grellscheid SN et al. Identification of novel androgen-regulated pathways and mRNA isoforms through genome-wide exon-specific profiling of the LNCaP transcriptome. PLoS ONE 2011; 6: e29088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res 2007; 67: 10455–10465. [DOI] [PubMed] [Google Scholar]
- Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell Stress Chaperones 2002; 7: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int 2005; 95: 1320–1326. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Morishima Y, Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem 2008; 283: 22885–22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst 2001; 93: 1687–1697. [DOI] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT. A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther 2005; 312: 546–553. [DOI] [PubMed] [Google Scholar]
- Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J Mol Endocrinol 2005; 35: 547–555. [DOI] [PubMed] [Google Scholar]
- Montero JC, Seoane S, Ocana A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res 2011; 17: 5546–5552. [DOI] [PubMed] [Google Scholar]
- Liu Y, Karaca M, Zhang Z, Gioeli D, Earp HS, Whang YE. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene 2010; 29: 3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin L, Frasson M, Donella-Deana A, Frezzato F, Pagano MA, Tibaldi E et al. Geldanamycin-induced Lyn dissociation from aberrant Hsp90-stabilized cytosolic complex is an early event in apoptotic mechanisms in B-chronic lymphocytic leukemia. Blood 2008; 112: 4665–4674. [DOI] [PubMed] [Google Scholar]
- Barouch-Bentov R, Che J, Lee CC, Yang Y, Herman A, Jia Y et al. A conserved salt bridge in the G loop of multiple protein kinases is important for catalysis and for in vivo Lyn function. Mol Cell 2009; 33: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen AM, Wallace ME, Goradia A, Jones SA, Croom HA, Metcalf D et al. A kinase-dead allele of Lyn attenuates autoimmune disease normally associated with Lyn deficiency. J Immunol 2009; 182: 2020–2029. [DOI] [PubMed] [Google Scholar]
- Araujo JC, Trudel GC, Paliwal P. Long-term use of dasatinib in patients with metastatic castration-resistant prostate cancer after receiving the combination of dasatinib and docetaxel. Cancer Manage Res 2013; 6: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007; 110: 4055–4063. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT et al. A quantitative analysis of kinase inhibitor selectivity. Nature Biotechnol 2008; 26: 127–132. [DOI] [PubMed] [Google Scholar]
- Yokota A, Kimura S, Masuda S, Ashihara E, Kuroda J, Sato K et al. INNO-406, a novel BCR-ABL/Lyn dual tyrosine kinase inhibitor, suppresses the growth of Ph+ leukemia cells in the central nervous system, and cyclosporine A augments its in vivo activity. Blood 2007; 109: 306–314. [DOI] [PubMed] [Google Scholar]
- Kimura S, Naito H, Segawa H, Kuroda J, Yuasa T, Sato K et al. NS-187, a potent and selective dual Bcr-Abl/Lyn tyrosine kinase inhibitor, is a novel agent for imatinib-resistant leukemia. Blood 2005; 106: 3948–3954. [DOI] [PubMed] [Google Scholar]
- Kasahara K, Nakayama Y, Ikeda K, Fukushima Y, Matsuda D, Horimoto S et al. Trafficking of Lyn through the Golgi caveolin involves the charged residues on alphaE and alphaI helices in the kinase domain. J Cell Biol 2004; 165: 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M, Bishop JL, Nip KM, Zardan A, Takeuchi A, Cordonnier T et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res 2013; 73: 3109–3119. [DOI] [PubMed] [Google Scholar]
- Zoubeidi A, Zardan A, Wiedmann RM, Locke J, Beraldi E, Fazli L et al. Hsp27 promotes insulin-like growth factor-I survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. Cancer Res 2010; 70: 2307–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux F, Thomas C, Yin MJ, Kuruma H, Beraldi E, Fazli L et al. Clusterin inhibition using OGX-011 synergistically enhances Hsp90 inhibitor activity by suppressing the heat shock response in castrate resistant prostate cancer. Cancer Res 2011; 71: 5838–5849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.