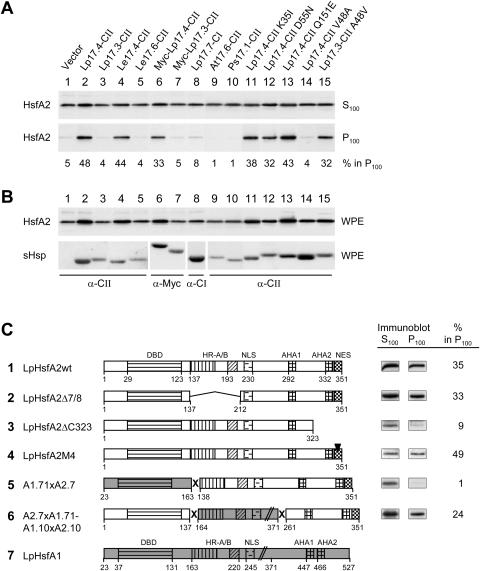
Figure 3.
Influence of different sHsps and functional domains of HsfA2 on the formation of soluble and sedimentable forms of HsfA2. HsfA2 was expressed in tobacco protoplasts together with the indicated sHsps. Whole protoplast extracts (WPE) in high salt and detergent buffer were centrifuged for 1 h at 100,000g, and the distribution of HsfA2 between supernatant (S100) and pellet fractions (P100) was estimated by immunoblot analysis. A, Numbers given at the bottom of the corresponding immunoblots represent the relative proportion of HsfA2 in the sedimentable form (S100 + P100 = 100%). B, Expression controls of HsfA2 and sHsps detectable in immunoblots of the WPE fractions. C, LpHsp17.4-CII was coexpressed with the indicated wild-type (sample 1) and mutant forms (samples 2–4) of HsfA2 as well as fusion proteins of HsfA2 with HsfA1 (5 and 6). Analysis of soluble (S100) and insoluble forms (P100) of the indicated Hsfs was performed and the signals of the immunoblot analyses as well as values for the amount of sedimentable HsfA2 calculated from the corresponding densitometer scans are given at the right. The block diagrams represent functional domains of HsfA1 (7, shaded) or HsfA2 (DBD, DNA-binding domain; HR-A/B oligomerization domain; NLS nuclear localization sequence; AHA1, 2, transcriptional activator motifs; NES, nuclear export signal).