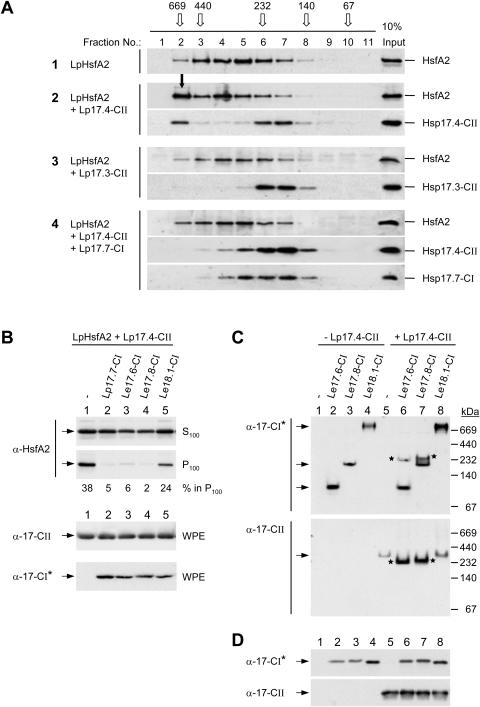
Figure 5.
Influence of class CI sHsps on the formation of HsfA2 and sHsp class CII complexes. A, HsfA2 was expressed either alone (sample 1) or in the indicated combinations with sHsps (samples 2–4) in tobacco protoplasts and separated by size exclusion chromatography (SEC) of whole protoplast extracts. The elution profiles were analyzed by immunoblotting as described in “Materials and Methods”. For comparison, immunoblot signals corresponding to one-tenth of the SEC loading samples are shown in the right lane. The elution profile and size of molecular mass standards are given on top (open arrows). The full arrow (sample 2, fraction no. 2) points to the signal corresponding to the sedimentable HsfA2 formed in presence of Hsp17.4-CII. B, The indicated isoforms of tomato class CI sHsps (samples 2–5) were coexpressed with LpHsfA2 and LpHsp17.4-CII and the formation of sedimentable HsfA2 was analyzed as described in Figures 3 and 4. C, Separation of native oligomeric sHsp complexes formed by the indicated isoforms of tomato Hsp17-CI in absence (−) or presence (+) of LpHsp17.4-CII in polyacrylamide pore exclusion gels. Arrows point to specific positions of homooligomeric complexes of the individual Hsp17-CI isoformes (first section) or of Hsp17.4-CII (second section). Asterisks indicate the positions of comigrating Hsp17-CI and -CII complexes in the coexpression samples (6 and 7). Separation and sizes of molecular mass standards are indicated at the right margin. D, For expression control aliquots of the samples indicated in part C were separated by SDS-PAGE and processed for immunoblot analysis as described in “Materials and Methods”. Because of an unequal detection of the Hsp17-CI isoforms by the peptide-specific antibody, the class CI-specific polyclonal antiserum generated in guinea-pigs (α-Hsp17-CI*, see “Materials and Methods”) was used for the corresponding immunodetections in parts B, C, and D.