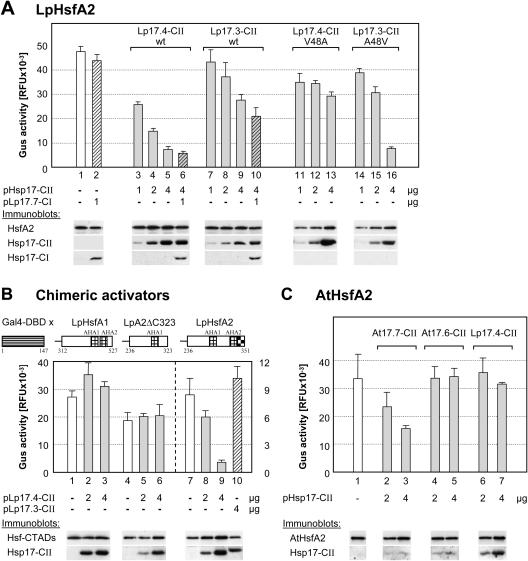
Figure 7.
Influence of sHsps on the activator function of HsfA2. A, Hsf-dependent reporter assay with phsp17gus as reporter. To achieve optimum activity, 50,000 tobacco protoplasts were transformed with 2 μg of HsfA2 expression plasmid plus 0.2 μg of HsfA1ΔC394 expression plasmid (sample 1) and for samples 2 to 16 the indicated amounts of sHsp encoding plasmids were added to the transformation mix. Samples 11 to 13 contain the LpHsp17.4-CII V48A mutant, whereas samples 14 to 16 contain the LpHsp17.3-CII A48V mutant (for sequence details, see Fig. 1). Expression levels of the proteins were controlled by immunoblot analysis as indicated at the bottom. The GUS activity was determined in cell lysates harvested 11 h after transformation (see “Materials and Methods”). B, The pGal4DBDxgus reporter was used to monitor the activator potential of Gal4DBD fusion proteins with the indicated activation domains of tomato HsfA1, HsfA2ΔC323, and HsfA2. Note the differences in the activity scales for the first two activators (left) as compared to the Gal4DBDxHsfA2 activator (right). C, Hsf-dependent reporter assay of Arabidopsis AtHsfA2 in tobacco protoplasts. One microgram of AtHsfA2 expression plasmid was transformed alone (no. 1) or in mixtures with the indicated amounts of plasmids encoding the two isoforms of class CII sHsps from Arabidopsis (nos. 2–5) and the HsfA2-interacting Hsp17.4-CII of tomato (nos. 6, 7). The AtHsfA2 expression plasmid and the immunserum for detection of the protein were kindly provided by P. von Koskull-Döring (unpublished data).