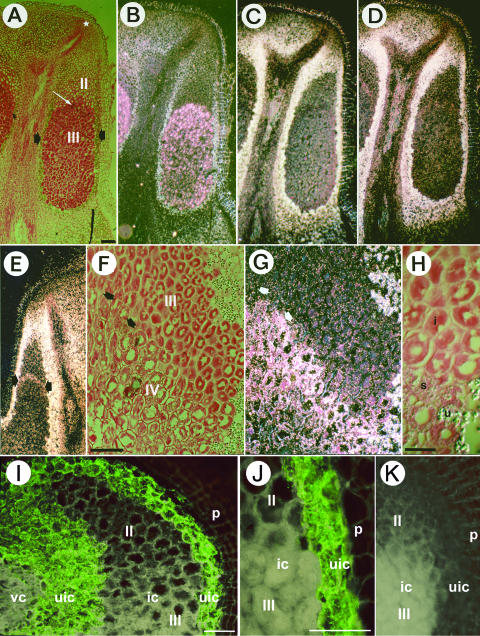
Figure 3.
In situ localization of DgGS1-COD, DgGS1-UTR, and nifH mRNA; and immunolocalization of GS protein in longitudinal tissue sections of D. glomerata root nodules. A to I, In situ localization of mRNA. A, F, and H are brightfield images in which silver grains denoting hybridization are visible as black dots. B, C, D, E, and G are darkfield images in which silver grains appear as white or pinkish-white dots. Note that the cell walls of periderm and uninfected cortex (B) are birefringent in the polarized-light optics, as well as starch grains in the uninfected cortex. Scale bar in A represents 500 μm (valid for A to E). Scale bar in F represents 200 μm (valid for F and G). Scale bar in H represents 100 μm. A to D show a series of adjacent sections hybridized with different antisense RNA probes. A and B, Hybridization with Frankia nifH. The nodule lobe meristem in A is marked by a white star. Short black arrows point to the layers of uninfected cells containing large starch grains that surround the zone of infected cells. The white arrow points to an infected cell in the zone where nitrogen fixation was first detected, as denoted by Frankia nifH expression. The infection zone (II) and the nitrogen fixation zone (III) of the infected area are labeled in A. C, Hybridization with part of the coding region of D. glomerata Gln synthetase (DgGS1-COD). No signal above background was found in the nodule meristem, in the infected cells or in the vascular system of the nodule. High DgGS1-1 expression was detected in the uninfected cells surrounding the patches of infected cells. Periderm birefringence due to epipolarization indicates unlabeled background for nodule tissues including uninfected cortex. Unlabeled background levels can also be seen by examining birefringent tissues in B, or the lignified (birefringent) xylem elements in C and D, which are dead cells lacking gene expression. D, Hybridization with the 3′ UTR of D. glomerata Gln synthetase (DgGS1-UTR). No significant difference was observed between the expression patterns of the two probes. E, Hybridization with DgGS1-UTR. Expression is detected mainly in the uninfected cortical tissue as in D. In some nodules, very low levels of DgGS1-1 expression were detectable in infected cells at the onset of nitrogen fixation, as shown in E. F and G, Hybridization with DgGS1-COD. Detail of the older part of a nodule where some senescence of infected cells was observed. The nitrogen fixation zone (III) and the zone of senescence (IV) of infected cells are labeled. Expression of DgGS is found in the senescent infected cells (arrows). At the upper right in F and G, large starch grains are visible in uninfected cortical cells adjacent to the infected zone; smaller starch grains are present in the senescent infected cells. H, Detail of F Nitrogen-fixing infected (i), senescent infected (s), and uninfected (u) cells can be distinguished. I to L, GS protein immunolocalized in D. glomerata root nodules. I, In nodule tissue, the strong green FITC-fluorescence signal indicating the presence of GS was exclusively observed in uninfected cells (uic). GS was absent in immature (zone II) and mature (zone III) infected cells (ic), in the periderm (p) and in central vascular cylinder (vc). Scale bar in I represents 100 μm, valid for I and K. J, Detail of infected (ic; zone III) and peripheral uninfected (uic) nodule parenchyma cells. The green FITC-fluorescence signal is restricted to uninfected cells. Scale bar in J represents 50 μm. K, Control. The green FITC-fluorescence signal is absent when the primary antibody is replaced by phosphate-buffered saline (PBS).