Abstract
Insulin maintains whole body blood glucose homeostasis, in part, by regulating the amount of the GLUT4 glucose transporter on the cell surface of fat and muscle cells. Insulin induces the redistribution of GLUT4 from intracellular compartments to the plasma membrane, by stimulating a large increase in exocytosis and a smaller inhibition of endocytosis. A considerable amount is known about the molecular events of insulin signaling and the complex itinerary of GLUT4 trafficking, but less is known about how insulin signaling is transmitted to GLUT4 trafficking. Here, we show that the AS160 RabGAP, a substrate of Akt, is required for insulin stimulation of GLUT4 exocytosis. A dominant-inhibitory mutant of AS160 blocks insulin stimulation of exocytosis at a step before the fusion of GLUT4-containing vesicles with the plasma membrane. This mutant, however, does not block insulin-induced inhibition of GLUT4 endocytosis. These data support a model in which insulin signaling to the exocytosis machinery (AS160 dependent) is distinct from its signaling to the internalization machinery (AS160 independent).
INTRODUCTION
Insulin controls glucose uptake into fat and muscle cells by acutely regulating the amount of the GLUT4 glucose transporter on the plasma membrane (Bryant et al., 2002). In basal adipocytes, GLUT4 is retained by a multistep process involving slow exocytosis and rapid endocytosis (Holman et al., 1994; Yeh et al., 1995; Karylowski et al., 2004). The intracellular trafficking route taken by GLUT4 is specialized, with IRAP, a transmembrane aminopeptidase of unknown physiological function, the only other protein known to traffic by the same pathway (Ross et al., 1997; Garza and Birnbaum, 2000). The intracellular itinerary of GLUT4 is complex, with GLUT4 rapidly cycling directly between endosomes that contain the TR and a specialized intracellular compartment that contains IRAP but excludes the TR (Martin et al., 2000; Zeigerer et al., 2002; Karylowski et al., 2004). Insulin increases GLUT4 exocytosis and partially inhibits its internalization, resulting in a net redistribution of GLUT4 to the plasma membrane.
The precise mechanism by which insulin signals to GLUT4 trafficking is not known (Saltiel and Pessin, 2002). One crucial step is insulin activation of the class Ia phosphoinositide (PI) 3-kinase (Hausdorff et al., 1999). The lipid products of PI 3-kinase activate the phosphoinositide-dependent protein kinase 1, which in turn activates the serine/theronine kinase Akt (protein kinase B). Inhibition of Akt activity with interfering antibodies, substrate peptides, dominant-negative mutants, or knockdown by small interfering RNA inhibit GLUT4 redistribution to the cell surface (Kohn et al., 1998; Hill et al., 1999; Jiang et al., 2003). Analysis of adipocytes lacking Akt-2 supports a prominent role for Akt in insulin regulation of GLUT4 translocation (Bae et al., 2003). Constitutively active Akt induces a translocation of GLUT4 to the cell surface, suggesting that activation of Akt is sufficient for insulin's effect on glucose uptake (Kohn et al., 1996; Foran et al., 1999).
A substrate of Akt has been identified that may provide a link between insulin signaling and GLUT4 trafficking. This protein, AS160, which has a GAP homology domain, is phosphorylated by Akt in response to insulin (Kane et al., 2002). GAP proteins are involved in regulating the GTP/GDP cycle of the Rab protein family, GTPases that regulate numerous membrane trafficking processes (Deneka et al., 2003). AS160-4P, a construct in which four of the six potential Akt phosphorylation sites have been mutated, partially inhibits insulin-induced GLUT4 redistribution to the surface of adipocytes (Sano et al., 2003). AS160-4P mutated in the arginine predicted to be essential for AS160 GAP activity, does not inhibit GLUT4 translocation, providing evidence that phosphorylation of AS160 inactivates the GAP activity and that the inhibition of GAP activity is required for translocation. To date, the Rab protein regulated by AS160-GAP has not been identified.
Here, we used AS160-4P as a reagent to further characterize how insulin signal transduction intersects with GLUT4 trafficking. We found that AS160-4P blocked the effect of constitutively active Akt on GLUT4 trafficking, which provides strong evidence that AS160 acts downstream of Akt. AS160-4P completely inhibited the stimulatory effect of insulin on GLUT4 exocytosis, which accounts for its inhibition of insulin-regulated redistribution of GLUT4 to the cell surface. We also show that AS160-4P blocks insulin-stimulated exocytosis of GLUT4 at a step preceding GLUT4-containing vesicles docking and fusing with the plasma membrane. Interestingly, AS160-4P did not affect insulin's inhibition of GLUT4 internalization, demonstrating that the insulin signal cascade that regulates GLUT4 internalization is independent of AS160 and therefore distinct from signaling to GLUT4 exocytosis, which is dependent on phosphorylation of AS160.
MATERIALS AND METHODS
Ligands and Chemicals
Anti-hemagglutinin epitope (HA) antibody (HA.11) was purchased as ascites (Covance, Berkley, CA) and purified on a protein G affinity column (Amersham Biosciences AB, Uppsala, Sweden). The concentration of anti-HA antibody required to saturate the HA-epitope of HA-GLUT4-GFP was determined for each preparation of antibody by measuring cell-associated anti-HA antibody in a 10-min pulse at 37°C. Typically, the saturating concentration of HA.11 was 50 μg/ml. HA.11 was labeled with fluorescent dye Cy3 (Biological Detection Systems, Pittsburgh, PA), according to the manufacturer's instructions. Human transferrin was purchased from Sigma-Aldrich (St. Louis, MO) and conjugated to horseradish peroxidase (HRP) or fluorescent dyes as described previously (Lampson et al., 2000). Fluorescent antibodies were purchased from Jackson Immunolabs, Inc. (West Grove, PA). All chemicals were purchased from Sigma Inc. (St. Louis, MO).
Cell Culture
3T3-L1 fibroblasts were cultured, differentiated, and electroporated as described previously (Zeigerer et al., 2002). Cells were used for experiments 24–48 h after electroporation.
Fluorescence Quantification
Fluorescence microscopy was performed with a DMIRB inverted microscope (Leica, Deerfield, IL), with a cooled charge-coupled device camera (Princeton Instruments, West Chester, PA). All images were collected with a 40 × 1.25 NA oil immersion objective. MetaMorph software (Universal Imaging, West Chester, PA) was used for image processing and quantification as described previously (Lampson et al., 2001; Zeigerer et al., 2002; Karylowski et al., 2004).
Total Internal Reflection Fluorescence (TIRF) Microscopy
Adipocytes were incubated in serum-free DMEM medium with 220 mM bicarbonate and 20 mM HEPES, pH 7.4, for 3 h at 37°C in 5% CO2/air (medium 1). Insulin (170 nM, used at all times) was added to one-half the dishes for the last 15 min. Cells were fixed in 3.7% formaldehyde, and the insulin-induced GFP redistribution was determined using a total internal reflection fluorescence microscope. The TIRF microscope has been described previously (Moskowitz et al., 2003). A 60 × 1.45 NA objective (Olympus America, Melville, NY) was used to perform “prism-less” TIRF. The evanescent field decay length was 100–250 nm with this objective, with a pixel size of 112 × 112 nm2 in the acquired images. Cells expressing HA-GLUT4-GFP were identified by GFP in epifluorescence mode of the microscope. Both epifluorescence and TIRF images of cells were acquired. MetaMorph software was used to quantify the images. The GFP fluorescence in the TIRF mode was divided by the GFP epifluorescence intensity, normalizing the TIRF fluorescence for the total HA-GLUT4-GFP expressed per cell. The TIRF fluorescence is a measure of the HA-GLUT4-GFP within the evanescent excitation field (∼250 nm from dorsal membrane). All images were corrected for background fluorescence measured in cells that did not express HA-GLUT4-GFP.
Trafficking Assays
The insulin-induced redistribution of HA-GLUT4-GFP and TR was determined as described previously (Lampson et al., 2001; Zeigerer et al., 2002; Karylowski et al., 2004). Briefly, surface HA-GLUT4-GFP per cell was determined in indirect immunofluorescence with HA.11 and a Cy3-labeled secondary antibody, whereas the GFP fluorescence was a measure of total HA-GLUT4-GFP per cell. The total amount of the TR expressed was determined by uptake of Cy3-transferrin (Tf) from the medium in a 4-h incubation at 37°C. The amount of the construct on the surface was detected with an antibody against the extracellular domain of the human TR.
For translocation experiments with HA-GLUT4-GFP and myr-Akt1, cells were incubated with saturating concentration of a mouse-anti-HA antibody for 6 h to label all the HA-epitopes on the HA-GLUT4-GFP construct. Surface HA-GLUT4-GFP was detected in fixed and nonpermeabilized cells by staining with a Cy5-anti-mouse antibody. To establish that the cells coexpress the HA-tagged myr-Akt1, cells were then permeabilized with 250 μg/ml saponin and stained with a Cy3-labeled anti-HA antibody. Because the HA epitopes on HA-GLUT4-GFP were bound during the incubation at 37°C, only HA-epitopes on the myr-Akt1 were detected in the fixed, permeabilized cells.
The efflux rate constant measurements for HA-GLUT4-GFP were determined as described previously (Karylowski et al., 2004).
The internalization rate constant was measured by incubating cells with a saturating amount of the HA.11 antibody in medium 1 for various periods of time. To obtain measurements in the insulin state, cells were pretreated with insulin for 30 min, and the HA.11 antibody was internalized in the presence of insulin. Cells were fixed and the HA.11 on the cell surface was blocked with saturating amount of a goat anti-mouse IgG antibody (antibody #115-175-062; Jackson Immunoresearch Laboratories, West Grove, PA) labeled with Cy5. The cells were refixed, permeabilized with 250 μg/ml saponin, and stained with antibody #115-175-062 labeled with Cy3. The Cy3 antibody #115-175-062 only labels HA.11 internalized into cells. To derive an internalization rate constant it is necessary to ratio the amount of HA-GLUT4-GFP internalized to the amount on the cell surface by using the same fluorophor. The amount of HA-GLUT4-GFP on the cell surface was measured in separate dishes of identically treated cells by indirect imunofluorescence with HA.11 and Cy3-labeled antibody #115-175-062. The internal-to-surface ratio (Cy3/GFP) for each time point was fit to a straight line, the slope of which is the internalization rate constant.
Epitope Ablation Assay
The intracellular distribution of HA-GLUT4-GFP between endosomes and the specialized compartment was determined as described previously (Lampson et al., 2001; Zeigerer et al., 2002; Karylowski et al., 2004). Cells transiently transfected with HA-GLUT4-GFP and TR or IRAP-TR were incubated for 5 or 6 h in medium 1 with saturating concentration of HA.11 antibody, with or without 20 μg/ml HRP-transferrin. To determine the intracellular distribution in the insulin state cells were incubated with insulin for the last 0.5 h of incubation with HA.11 antibody and horseradish HRP-transferrin. The cells are then placed on ice, washed twice with cold medium 2 (150 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 5 mM KCl, 1 mM MgCl2, pH 7.4), and incubated for 30 min with 250 μg/ml diaminobenzidine (DAB) and 0.0025% H2O2 in the dark. The cells were washed twice with medium 2 and fixed with 3.7% formaldehyde for 10 min. HA.11 antibody on the surface was bound with saturating concentration of unlabeled donkey anti-mouse IgG antibody (antibody #715-005-150; The Jackson Laboratory, Bar Harbor, MN). The cells were refixed and the intracellular HA.11 was detected in saponin-permeabilized cells with a Cy3-labeled antibody #715-005-150. The total nonablated fluorescence was measured from cells that were not incubated with HRP-transferrin but treated with DAB and H2O2.
RESULTS
Constitutively Active PI 3-Kinase Is Not Sufficient to Induce Full Translocation of GLUT4
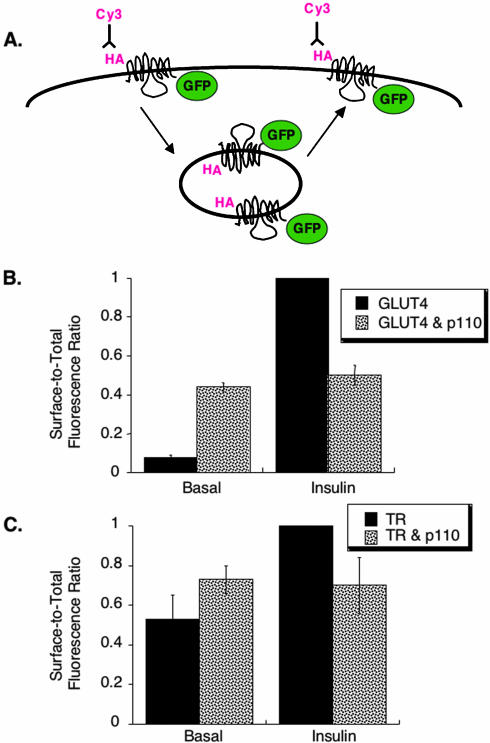
As a first step toward identifying the intersection between the signal transduction pathway and movement of GLUT4, we determined the effect of a constitutively active, membrane targeted form of the PI 3-kinase catalytic subunit p110caax on GLUT4 behavior (Egawa et al., 2000). The surface-to-total distribution of a reporter for GLUT4, HA-GLUT4-GFP, coexpressed in 3T3-L1 adipocytes with p110caax, was determined using single-cell, quantitative fluorescence microscopy (Zeigerer et al., 2002). The green fluorescent protein (GFP) on the carboxyl cytoplasmic domain is a measure of the total amount of HA-GLUT4-GFP expressed per cell, and the HA-epitope on the first exofacial loop is a measure of HA-GLUT4-GFP on the plasma membrane (Figure 1A). In unstimulated control cells, HA-GLUT4-GFP was predominantly retained within cells, whereas it was redistributed to the plasma membrane after a 15-min incubation with 170 nM insulin (Figure 1B). In unstimulated cells expressing p110caax, the basal surface-to-total ratio was ∼5 times that in that basal control cells, documenting that expression of p110caax promoted a redistribution of HA-GLUT4-GFP to the surface. However, the HA-GLUT4-GFP redistribution to the plasma membrane induced by expression of p110caax was only about one-half of that in insulinstimulated control cells (Figure 1B). Thus, expression of p110caax did not induce the full insulin-stimulated redistribution of GLUT4 to the cell surface. This observation is consistent with previous reports that activation of PI 3-kinase is not by itself sufficient to induce complete GLUT4 translocation (Isakoff et al., 1995; Wiese et al., 1995; Guilherme and Czech, 1998). Treatment of cells expressing p110caax with insulin did not induce a further increase of HA-GLUT4-GFP on the cell surface (Figure 1B), consistent with previous reports that expression of constitutively active p110caax inhibits insulin's action (Egawa et al., 1999). Although we do not know why p110caax inhibits insulin's action, it is likely that the constitutively active lipid kinase has numerous affects on cell physiology, beyond those specific to GLUT4 trafficking. Thus, although activation of PI 3-kinase is required for insulin-stimulated GLUT4 translocation, transient expression of constitutively active p110caax does not quantitatively recapitulate insulin's effect on GLUT4.
Figure 1.
Translocation assay of HA-GLUT4-GFP expressing constitutively active PI 3-kinase. (A) Schematic of the HA-GLUT4-GFP construct and the translocation assay (Lampson et al., 2000). Quantification of the HA-epitope on the cell surface, determined by indirect immunofluorescence with a Cy3-labeled secondary antibody, is a measure of the amount of HA-GLUT4-GFP inserted into the plasma membrane. (B) Summary of data from five matched experiments in adipocytes expressing HA-GLUT4-GFP or coexpressing HA-GLUT4-GFP and FLAG epitope-tagged p110caax (mean ± SEM). The data are normalized to the surface-to-total (Cy3/GFP) ratio in insulin-stimulated control cells. Insulin stimulation was with 170 nM for 15 min (used in all experiments). Coexpression of p110caax was determined with a fluorescent rabbit-anti-FLAG antibody. (C) Summary of data from four matched experiments in adipocytes expressing TR or coexpressing TR and p110caax (mean ± SEM). The data are normalized to the surface-to-total ratio in insulin-stimulated control cells. The effects of p110caax on TR surface-to-total ratios are not statistically significant.
We examined the effect of p110caax on the surface-to-total distribution of the transferrin receptor (TR), a marker of the general endocytic trafficking. Cell surface TR in unstimulated cells expressing p110caax tended to be increased relative to control cells, although this increase was not statistically significant (Figure 1C). As was the case for HA-GLUT4-GFP, insulin did not increase TR on the surface of cells expressing of p110caax.
Constitutively Active Akt1 Induces Translocation of GLUT4
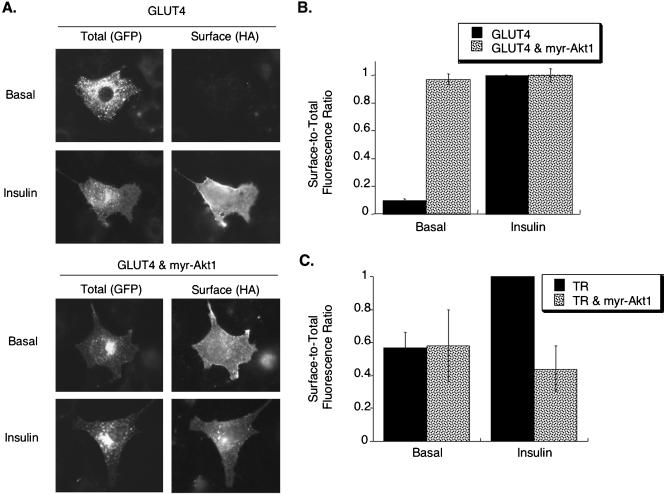
We next used a myristylated-Akt1 (myr-Akt1), which is constitutively active because it is targeted to the membrane, to determine whether constitutively active Akt mimics insulin's actions on membrane trafficking (Kohn et al., 1996; Foran et al., 1999). Myr-Akt1 induced a large shift of HA-GLUT4-GFP to the surface of adipocytes without causing a pronounced change in the morphology of the intracellular compartments that contain HA-GLUT4-GFP. In both control and myr-Akt1–expressing cells, the GFP pattern of HA-GLUT4-GFP was in punctate structures distributed throughout the cell as well as in structures concentrated in the perinuclear region (Figure 2A). Quantification of these data demonstrated that constitutively active Akt1 recruited HA-GLUT4-GFP to the plasma membrane to the same degree as insulin stimulation of control cells (Figure 2B). Treatment of cells expressing myr-Akt1 with insulin did not induce an increase in HA-GLUT4-GFP surface expression above that stimulated by myr-Akt1 alone, consistent with previous studies (Kohn et al., 1996; Foran et al., 1999).
Figure 2.
Myr-Akt1 stimulates translocation of GLUT4. (A) Cells expressing HA-GLUT4-GFP with and without myr-Akt1. Total HA-GLUT4-GFP patterns were determined by the GFP fluorescence, and HA-GLUT4-GFP on the surface was determined by indirect immunofluorescence of the HA epitope in fixed cells. Coexpression of HA-GLUT4-GFP and myr-Akt1 was confirmed by indirect immunofluorescence of fixed, detergent permeabilized cells. (B) Summary of data from six matched experiments in adipocytes expressing HA-GLUT4-GFP or coexpressing HA-GLUT4-GFP and Myr-Akt1 (mean ± SEM). The data are normalized to the surface-to-total (Cy3/GFP) ratio in insulin stimulated control cells. (C) Summary of data from five matched experiments in adipocytes expressing TR or coexpressing TR and myr-Akt1 (mean ± SEM). The surface-to-total TR ratios are normalized to the surface-to-total ratio in insulin-stimulated control cells.
The myr-Akt1 did not have an effect on the basal surface-to-total distribution of the TR, indicating that it specifically affected HA-GLUT4-GFP trafficking in unstimulated cells (Figure 2C). Myr-Akt1 inhibited insulin-stimulated recruitment of TR to the cell surface, demonstrating that although myr-Akt1 mimics insulin's action on GLUT4, it does not recapitulate the effect of insulin on general endosomal trafficking. Regardless, because myr-Akt1 mimicked insulin's effects on surface expression of GLUT4, we further examined the effects of myr-Akt1 on intracellular trafficking of HA-GLUT4-GFP.
Myr-Akt1 Alters Intracellular Distribution of GLUT4
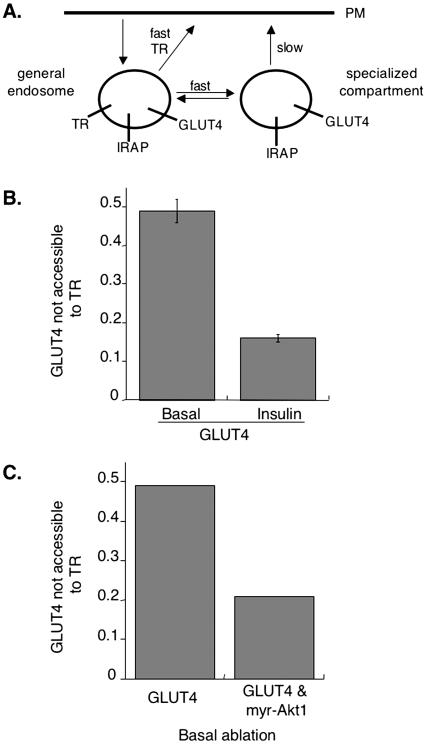
We have shown that a chimera, IRAP-TR, containing the cytoplasmic domain of IRAP fused to the transmembrane and extracellular domains of the TR traffics like GLUT4 when expressed in 3T3-L1 adipocytes (Subtil et al., 2000; Zeigerer et al., 2002). We have used cells coexpressing HA-GLUT4-GFP and the TR, or IRAP-TR, in an HRP compartment ablation method to examine the distribution of GLUT4 among intracellular compartments in intact 3T3-L1 adipocytes (Zeigerer et al., 2002; Karylowski et al., 2004). Briefly, cells were incubated at 37°C in medium containing an anti-HA epitope antibody (HA.11) and HRP-transferrin. HA-GLUT4-GFP, as it traffics to the cell surface, is bound by HA.11, which remains bound as HA-GLUT4-GFP traffics through intracellular compartments, thereby delivering HA.11 to the lumen of compartments that contain HA-GLUT4-GFP. In cells expressing the TR, HRP-transferrin is delivered to endosomes, whereas in cells expressing the IRAP-TR, HRP-transferrin is delivered to endosomes and the GLUT4-specialized compartment. After incubation with HRP-transferrin and HA.11, cells are incubated with DAB and H2O2 for 30 min on ice. In this reaction, HRP oxidizes DAB to form a dense polymeric matrix, which destroys epitopes and quenches fluorescence within compartments containing HRP. The degree of HA.11 ablation is determined by single-cell quantitative fluorescence microscopy (Lampson et al., 2001; Zeigerer et al., 2002; Karylowski et al., 2004). The difference in ablation between cells expressing IRAP-TR and TR represents the fraction of GLUT4 in the “specialized compartment” (that is, accessible to IRAP but not TR). To measure the overlap between GLUT4 and TR in the insulinstimulated state, insulin is included for the last 30 min of incubation with HA.11 and HRP-transferrin. In unstimulated control cells, GLUT4 is nearly equally distributed between endosomes that contain the TR and intracellular compartments that are not accessible to the TR (Figure 3B; Zeigerer et al., 2002; Karylowski et al., 2004). We refer to the latter pool of HA-GLUT4-GFP as the specialized compartment to distinguish it from HA-GLUT4-GFP in endosomes. In the presence of insulin, the intracellular distribution of HA-GLUT4-GFP is shifted toward endosomes, with ∼80% of intracellular HA-GLUT4-GFP found in compartments accessible to the TR (Figure 3B; Zeigerer et al., 2002; Karylowski et al., 2004). Thus, in addition to stimulating a redistribution of GLUT4 to the cell surface, insulin promotes a shift in the distribution of intracellular GLUT4 to TR-containing endosomes.
Figure 3.
Myr-Akt1 mimics insulin's effect on intracellular GLUT4. (A) Cartoon of GLUT4 trafficking. In basal cells, GLUT4 is equally distributed between endosomes and a specialized compartment because it rapidly cycles intracellularly between these compartments. Insulin simulated efflux of GLUT4 to the cell surface correlates with a redistribution of intracellular GLUT4 to endosomes (Karylowski et al., 2004). (B) Summary of 12 independent ablation experiments in 3T3-L1 adipocytes in the basal and insulin-stimulated state (means ± SEM). The nonablatable fraction is the amount of intracellular GLUT4 that is inaccessible to the TR expressed as a fraction of that accessible to IRAP-TR (Zeigerer et al., 2002; Karylowski et al., 2004). We refer to this nonablatable fraction as the pool of GLUT4 in the specialized compartment, as discussed in the introduction. (C) Results from a single experiment in cells coexpressing myr-Akt1 in the basal state. The data are from 25 cells for each condition.
Because constitutively active Akt1 quantitatively mimics the effect of insulin on GLUT4 translocation, we examined the effect of myr-Akt1 on GLUT4's intracellular distribution. Myr-Akt1 decreased the fraction of GLUT4 in the specialized compartment by the same amount as insulin did in control cells, shifting the intracellular distribution of HA-GLUT4-GFP to ∼80% in TR-containing endosomes (Figure 3C). Thus, myr-Akt1 increased surface expression of HA-GLUT4-GFP (Figure 2) and caused a redistribution of GLUT4 to endosomes, mimicking insulin's effects on GLUT4 trafficking.
AS160-4P Blocks Insulin-stimulated Translocation of GLUT4 to the Cell Surface
An AS160 construct in which of four of the six Akt phophorylation sites are mutated to alanines (AS160-4P) inhibits insulin-induced GLUT4 translocation, supporting a role for Akt phosphorylation of AS160 in GLUT4 translocation (Sano et al., 2003).
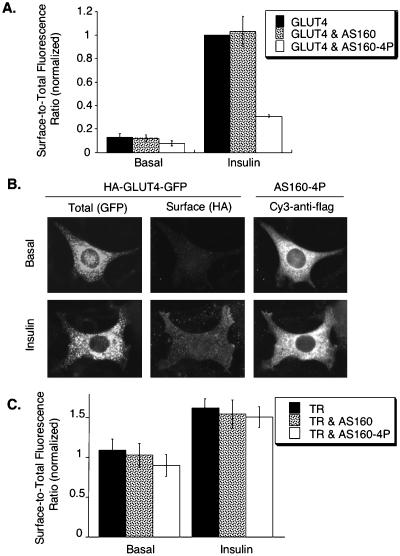
As a first step toward determining which step(s) of insulin-regulated GLUT4 trafficking is regulated by AS160, we examined the effect of wild-type and mutant AS160 on HA-GLUT4-GFP behavior in adipocytes. Overexpression of wild-type AS160 in adipocytes did not significantly alter the basal or insulin-stimulated surface-to-total distribution of HA-GLUT4-GFP, indicating that the amount of AS160 is not rate limiting for GLUT4 trafficking (Figure 4A). Thus, in agreement with a previous report, transient overexpression of AS160 did not affect the fold increase in surface expression of GLUT4 stimulated by insulin (Sano et al., 2003).
Figure 4.
AS160-4P blocks insulin-induced translocation of HA-GLUT4-GFP. (A) Summary of data from five matched independent experiments in cells expressing HA-GLUT4-GFP, HA-GLUT4-GFP, and AS160, or HA-GLUT4-GFP and AS160-4P (means ± SEM). The data are normalized to the surface-to-total ratio of HA-GLUT4-GFP in insulin-stimulated control cells. (B) Images from an adipocytes coexpressing HA-GLUT4-GFP and AS160-4P. Total HA-GLUT4-GFP patterns were determined by the GFP fluorescence, surface HA-GLUT4-GFP was determined by indirect immunofluorescence of the HA epitope in fixed cells, and expression of the FLAG-tagged AS160-4P was determined by indirect immunofluorescence after permeabilization of the fixed cells. (C) TR surface-to-total distributions in cells coexpressing AS160 or AS160-4P. The data are from a representative experiment (mean of 20 cells ± SEM).
Expression of AS160-4P did not significantly alter the morphology of intracellular compartments containing HA-GLUT4-GFP in basal or stimulated cells. However, the morphology of the GLUT4 compartments in 3T3-L1 adipocytes is heterogeneous; therefore, only gross changes in morphology would be evident. The overexpressed AS160-4P colocalized to some degree with compartments that contain HA-GLUT4-GFP, but AS160-4P was, for the most part, distributed throughout the cytosol (Figure 4B). Expression of AS160-4P inhibited insulin-stimulated redistribution of HA-GLUT4-GFP to the cell surface (Figure 4B). There was a slight decrease in the surface-to-total distribution of HA-GLUT4-GFP in unstimulated cells expressing AS160-4P, although this trend did not reach statistical significance, whereas the surface-to-total ratio of HA-GLUT4-GFP in the presence of insulin was reduced by ∼70% relative to control cells (Figure 4A). The magnitude of the effect of AS160-4P on the insulin-stimulated fold translocation of GLUT4 that we measured is in good agreement with a previous report (Sano et al., 2003). Overexpression of AS160-4P did not significantly alter the surface-to-total distribution of the TR (Figure 4C). This distribution is determined by the TR internalization and exocytosis rate constants, indicating that the expression of AS160-4P does not alter the TR endocytosis and recycling rate constants. Thus, these data indicate that the effect of AS160-4P is specific to GLUT4 trafficking. Interestingly, AS160-4P did not seem to blunt the small effect of insulin on TR surface-to-total distribution, indicating that insulin signaling to the TR is independent of AS160.
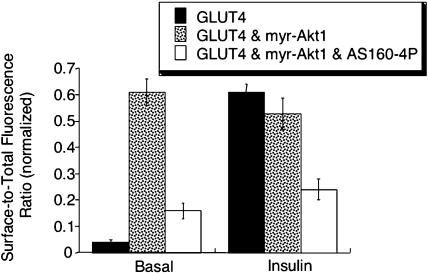
Because it is postulated that AS160 activity is modulated by insulin through the action of Akt, we next determined whether AS160-4P could inhibit the effects of myr-Akt1 on GLUT4. Expression of AS160-4P significantly reduced the surface-to-total distribution of HA-GLUT4-GFP in cells expressing myr-Akt1 (Figure 5). These data provide direct support for the hypothesis that AS160 is a downstream target of Akt required for insulin-regulated redistribution of GLUT4 to the cell surface.
Figure 5.
AS160 functions downstream of Akt. The surface-to-total HA-GLUT4-GFP distribution in adipocytes expressing HA-GLUT4-GFP, or HA-GLUT4-GFP and myr-Akt1, or HA-GLUT4-GFP and myr-Akt1 and AS160-4P are shown. The data are from a representative experiment (mean of 20 cells ± SEM).
AS160-4P Perturbs Intracellular GLUT4 Trafficking
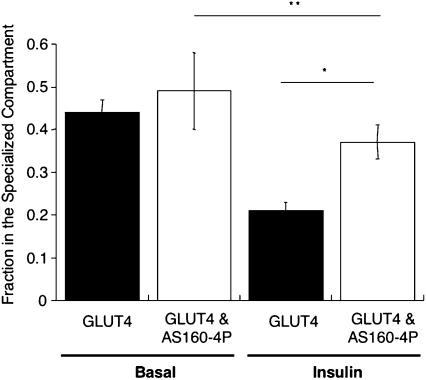
The insulin-stimulated increased expression of GLUT4 on the plasma membrane is dependent on mobilization of an intracellular pool of GLUT4; therefore, we next sought to determine the effect of AS160-4P on intracellular GLUT4 trafficking by using HRP-transferrin ablation method. AS160-4P did not change the intracellular distribution of GLUT4 between TR-containing endosomes and the specialized compartment in unstimulated cells, consistent with AS160-4P not significantly altering the surface-to-total ratio of HA-GLUT4-GFP in basal conditions (Figure 6). However, AS160-4P blocked the effect of insulin on the distribution of HA-GLUT4-GFP between these two compartments (Figure 6). Thus, AS160-4P inhibits insulin-stimulated redistribution of HA-GLUT4-GFP among intracellular compartments as well as its redistribution to the cell surface.
Figure 6.
AS160-4P blocks the effect of insulin on the intracellular HA-GLUT4-GFP. Ablation experiment with HA-GLUT4-GFP, IRAP-TR and the TR in the basal and insulin-stimulated state with or without expressing AS160-4P. The fraction in the specialized compartment is a measure of the amount of intracellular HA-GLUT4-GFP segregated away from the TR containing general endosomes. The data are the means ± SEM of five experiments. The data from the individual experiments were normalized to the Cy3/GFP ratio in control cells (no HRP-Tf uptake) for that experiment and the fraction in the specialized compartment is determined as described above. *p = 0.01; **p = 0.3.
AS160-4P Inhibits Insulin-stimulated HA-GLUT4-GFP Exocytosis
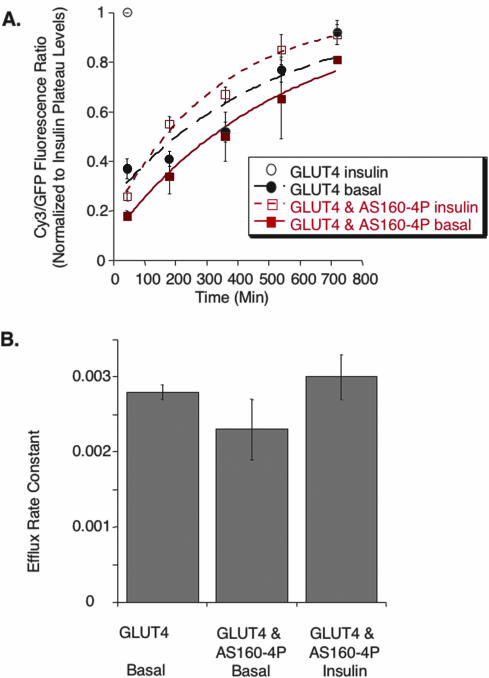
Insulin stimulates redistribution of GLUT4 to the cell surface predominantly by stimulating GLUT4 efflux from intracellular compartments to the cell surface. We next determined the effect of AS160-4P on HA-GLUT4-GFP exocytosis (Karylowski et al., 2004). Cells are incubated at 37°C in medium containing a saturating amount of HA.11. The accumulation of the antibody in cells is measured as a function of incubation time by indirect immunofluorescence of fixed, permeabilized cells. The fluorescence rises to a plateau level, at which time all the HA-GLUT4-GFP has been bound by HA.11. The exocytic rate constant determines the rise to the plateau level. In basal cells expressing AS160-4P, we measured an exocytic rate constant of ∼0.002 min-1 for HA-GLUT4-GFP, which is the not statistically different from the rate constant we measured for controls cells in the same experiments (Figure 7). In insulin-stimulated cells expressing AS160-4P, the HA-GLUT4-GFP exocytic rate constant was of 0.003 min-1, significantly less than the insulin-stimulated HA-GLUT4-GFP exocytosis rate constant of ∼0.08 min-1 that we have previously measured in control cells (Figure 7; Karylowski et al., 2004). These data directly demonstrate that AS160-4P blocks the effect of insulin on the exocytosis of GLUT4-containing vesicles, which accounts for blunted insulin-regulated redistribution of HA-GLUT4-GFP to the surface of cells expressing AS160-4P.
Figure 7.
AS160-4P inhibits insulin-stimulated exocytosis of HA-GLUT4-GFP. (A) Time-dependent uptake of a HA.11 anti-HA epitope antibody from the medium at 37°C. The Cy3/GFP ratios per time in each individual experiment were normalized to the insulin plateau level in that experiment. Uptake in the presence of insulin saturates by 45 min (single time point shown for control cells). The data are the average normalized Cy3/GFP ratios of five experiments ± SEM. The curves are (Cy3/GFP)t = 1 - exp(-ke*t); where (Cy3/GFP)t is the fraction of HA-GLUT4-GFP bound by anti-HA antibody at time t, and ke is the exocytic rate constant. (B) Summary of the exocytic rate constant of HA-GLUT4-GFP derived from the curves in A (means ± SEM). None of the differences are significant.
AS160-4P Inhibits the Insulin-stimulated Recruitment of the Intracellular Pool of GLUT4
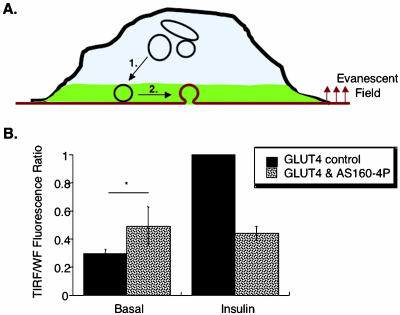
Insulin could increase exocytosis by stimulating a number of different steps of GLUT4 trafficking. For example, insulin could recruit GLUT4-containing vesicles to near the cell surface and/or increase fusion of these vesicles with the plasma membrane (Figure 8). Consequently, AS160-4P could inhibit insulin-stimulated exocytosis at a number of different steps. We used TIRF microscopy to further examine inhibition of translocation by AS160-4P. In this method only fluorophors within ∼250 nm of the dorsal membrane are excited (Moskowitz et al., 2003). When insulin-stimulated redistribution of HA-GLUT4-GFP-containing vesicles is monitored by GFP fluorescence in TIRF, no distinction is made between HA-GLUT4-GFP inserted into the membrane from HA-GLUT4-GFP in vesicles that are adjacent to, but not fused with, the membrane (Figure 8). Thus, unlike translocation measured by the exposure of the HA epitope on the surface, which is dependent on the fusion of vesicle with the plasma membrane, the TIRF assay will detect vesicles-containing GLUT4 that have been redistributed to within ∼250 nm of the membrane, regardless of whether they have fused with the membrane.
Figure 8.
AS160-4P inhibits insulin-induced recruitment of intracellular GLUT4. (A) Schematic of the TIRF translocation assay. Only the HA-GLUT4-GFP in the evanescent excitation field (green) is excited. GLUT4 movement to the cell surface involves transport of the intracellular pool of GLUT4 to the membrane (step 1) and the docking and fusion of these vesicles with the cell surface (step 2). (B) Summary of four independent experiments measuring HA-GLUT4-GFP translocation with TIRF microscopy assay (means ± SEM). The measured TIRF GFP fluorescence represents translocation of GFP to within 250 nm of the dorsal membrane. The TIRF GFP fluorescence is normalized to the total amount of HA-GLUT4-GFP per cell measured in the epifluorescence/wide field mode of the microscope (WF). The data are normalized to the insulin stimulated level. * p = 0.24.
In control cells, we found an insulin-stimulated threefold translocation of HA-GLUT4-GFP in the TIRF microscopy assay (Figure 8). This degree of translocation is similar to the magnitude of translocation previously reported using TIRF microscopy (Tengholm and Meyer, 2002). AS160-4P inhibited insulin-stimulated HA-GLUT4-GFP redistribution as measured by TIRF microscopy, indicating that AS160-4P inhibits translocation at a step preceding fusion, by blocking the redistribution of HA-GLUT4-GFP–containing vesicles from deeper inside cells to within ∼250 nm of the membrane (Figure 8, step 1).
Insulin Regulation of GLUT4 Internalization Is Independent of AS160
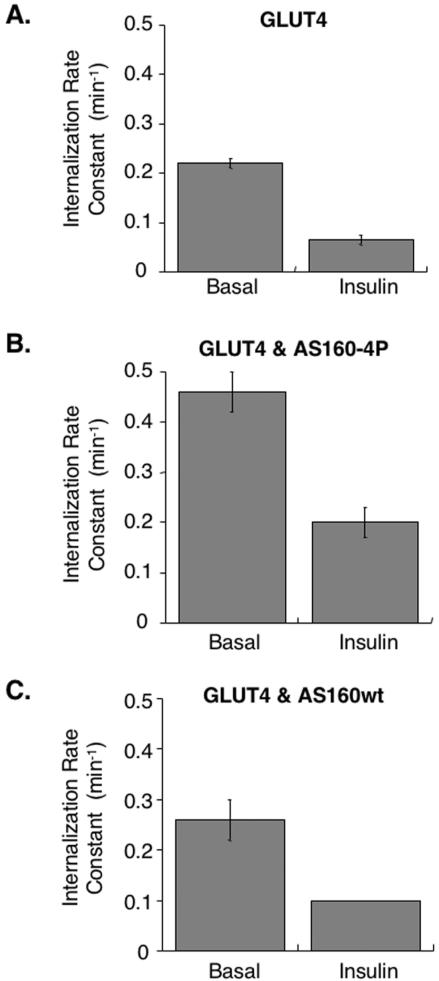
Although the principle mode of action on GLUT4 trafficking is stimulation of exocytosis, insulin has a smaller inhibitory effect on GLUT4 internalization (Jhun et al., 1992; Czech and Buxton, 1993). AS160-4P blocked insulin's effect on exocytosis, but it only partially inhibited HA-GLUT4-GFP translocation to the cell surface (Figure 4). One explanation for the residual translocation is that the insulin-regulated inhibition of GLUT4 internalization is not inhibited in cells expressing AS160-4P. Therefore, we next measured the effect of insulin on GLUT4 internalization in control and 3T3-L1 adipocytes expressing AS160-4P. In control cells, HA-GLUT4-GFP in the basal state was internalized with a rate constant of 0.2 ± .01 min-1, whereas in the presence of insulin, the rate constant was reduced by approximately threefold (Figure 9A). The magnitude of the inhibition by insulin is in agreement with the results of previous studies (Czech and Buxton, 1993; Yang and Holman, 1993). The HA-GLUT4-GFP internalization rate constant in unstimulated cells is similar to values reported for the TR, consistent with GLUT4 being internalized through clathrin-coated pits in basal conditions.
Figure 9.
Insulin regulation of GLUT4 internalization is independent of AS160. (A) HA-GLUT4-GFP internalization rate constant in basal and insulin-treated cells (means ± SEM, n = 4). (B) HA-GLUT4-GFP internalization rate constant in basal and insulin-treated cells coexpressing AS160-4P (means ± SEM, n = 5). (C) HA-GLUT4-GFP internalization rate constant in basal and insulin-treated cells coexpressing wild-type AS160 (basal data, mean ± SD, n = 2). Insulin data are from a representative experiment.
Overexpression of AS160-4P did not block the effect of insulin on GLUT4 internalization because insulin caused a two-to threefold reduction in HA-GLUT4-GFP internalization rate constant in cells expressing AS160-4P, indicating that insulin still regulates internalization when AS160-4P is expressed (Figure 9B). The insulin-regulated decrease in internalization can account for the residual insulin-stimulated redistribution of HA-GLUT4-GFP to the surface in cells expressing AS160-4P (Figure 5). These data indicate that insulin modulates internalization by a mechanism independent of AS160 activity and therefore that insulin signaling to the exocytosis machinery (AS160-dependent) is distinct from its signaling to the internalization machinery (AS160 independent).
For unknown reasons, expression of AS160-4P increased, relative to controls, both the basal and insulin-stimulated internalization rate constants of HA-GLUT4-GFP. This effect was specific for AS160-4P, because overexpression of wild-type AS160 did not change the internalization rate constants for GLUT4, in basal or insulin-stimulated conditions, compared with control cells (Figure 9C). Therefore, AS160-4P affects GLUT4 endocytosis in both basal and stimulated cells, without affecting insulin regulation of GLUT4 internalization. This effect is specific to GLUT4 because AS160-4P did not change the trafficking of the TR (Figure 4).
DISCUSSION
Here, we have characterized the intersection of insulin signal transduction and GLUT4 trafficking by using quantitative fluorescence microscopy methods (Figure 10). We show, in direct quantitative measurement of the endocytosis rate constant of GLUT4, that insulin inhibits GLUT4 internalization by approximately threefold. Although the effect of insulin on the internalization of GLUT4 is considerably smaller than its stimulation of GLUT4's exocytosis, the threefold reduction in internalization contributes to the overall redistribution of GLUT4 to the cell surface. Therefore, internalization, like exocytosis, is an important site of insulin regulation of GLUT4 trafficking. A question not addressed by this current study, is whether the effect of insulin on internalization is specific for GLUT4 or whether endocytosis of other proteins also is inhibited. Our findings are in agreement with studies that found, by using indirect measurements of internalization, insulin inhibition of uptake of GLUT4 (Jhun et al., 1992; Czech and Buxton, 1993; Yang and Holman, 1993).
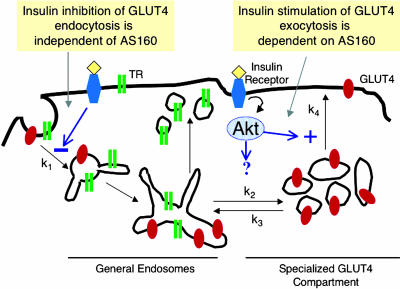
Figure 10.
Model for GLUT4 trafficking in 3T3-L1 adipocytes. GLUT4 is dynamically retained within cells in the basal state because it is internalized much faster than it is exocytosed. Insulin induces GLUT4 redistribution to the plasma membrane by increasing exocytosis and inhibiting internalization. Insulin signaling to GLUT4 exocytosis requires Akt phosphorylation of the AS160 RabGAP. Insulin signaling to endocytosis does not require AS160, demonstrating divergent signaling pathways regulate these two aspects of GLUT4 trafficking.
Previous studies have examined insulin signaling to GLUT4 trafficking by measuring the steady-state amount of GLUT4 on the cell surface. The amount of GLUT4 on the surface is determined by exocytosis and endocytosis, both of which are regulated by insulin. Therefore, to decipher how insulin signaling regulates the steady-state distributions of GLUT4, it is necessary to understand how the signal is transmitted to the individual steps of GLUT4 trafficking. Our findings that insulin regulates exocytosis and endocytosis by distinct mechanisms, underscore the importance of measuring the parameters that determine the amount of GLUT4 on the surface is studies of signaling to GLUT4 trafficking.
We focused on understanding the role of AS160, a downstream effector of Akt, in GLUT4 translocation. Of known proteins along the PI 3-kinase–dependent pathway that are believed to be specifically involved in GLUT4 translocation, AS160 is the furthest downstream from the insulin receptor. By studying the most “receptor distal step,” we hoped to focus on signaling required for GLUT4 movement and to minimize changes in GLUT4 behavior due to nonspecific effects. Expression of AS160-4P was known to inhibit insulin-stimulated increase of GLUT4 on the cell surface, although which step(s) of GLUT4 trafficking affected by AS160-4P were not examined (Sano et al., 2003). Here, in quantitative studies, we show that the basal and insulin-stimulated surface-to-total distributions of HA-GLUT4-GFP were unaffected by overexpression wild-type AS160, indicating that the amount of AS160 is not rate limiting for GLUT4 trafficking in either conditions. Expression of AS160-4P inhibited insulin-induced GLUT4 redistribution to the plasma membrane without significantly altering the basal surface-to-total distribution of GLUT4. Our finding that AS160-4P inhibits the effect of constitutively active myr-Akt1 on GLUT4 behavior provides compelling evidence that AS160 is a specific downstream effector of Akt in insulin regulation of GLUT4 traffic. Neither wild-type nor AS160-4P alters the behavior of the TR, suggesting that AS160-4P's effects are specific for the specialized, insulin-regulated pathway.
We examined individual steps of GLUT4 trafficking to map the step(s) blocked by AS160-4P. We found that AS160-4P abolishes insulin stimulation of GLUT4's exocytosis rate constant, even though in these cells insulin stimulated an approximately threefold redistribution of GLUT4 to the plasma membrane (Figure 4). The residual insulin-stimulated redistribution of GLUT4 to the surface of cells is because AS160-4P expression does not block insulin's effect on GLUT4 internalization. In AS160-4P–expressing cells, insulin inhibited GLUT4 internalization to nearly the same degree as in control cells. Therefore, AS160-4P interrupts signal transduction to GLUT4 exocytosis but not signaling to endocytosis.
AS160-4P, in addition to inhibiting GLUT4 movement to the plasma membrane, blocked the insulin-induced shift in the GLUT4 from a specialized intracellular compartment to TR-containing endosomes. Currently, we do not know whether insulin directly regulates the intracellular cycling of GLUT4 between endosomes and the specialized compartment, or whether this change is a consequence of increased exocytosis to the cell surface, and the AS160-4P results do not distinguish between these possibilities.
We propose that AS160-4P interrupts stimulation of GLUT4 redistribution to the cell surface by affecting the mobilization of GLUT4 at a step before vesicle docking/fusion with the plasma membrane (Figure 10, k4). Previous electron microscopy studies of fat cells have shown that GLUT4 vesicles accumulate in the perinuclear region near the Golgi, a site removed from the plasma membrane (Slot et al., 1991; Martin et al., 2000). Little GLUT4 was observed within ∼250 nm of the plasma membrane in basal conditions, whereas in insulin-stimulated cells there is an increase in GLUT4 at the plasma membrane as well as in small vesicles within 250 nm of the membrane (Martin et al., 2000). Live cell imaging studies also support the idea that one site of insulin action is to mobilize GLUT4 from perinuclear compartments that are not adjacent to the plasma membrane (Patki et al., 2001; Semiz et al., 2003). Our hypothesis that AS160-4P blocks recruitment of GLUT4 at a step before GLUT4 vesicles encounter the plasma membrane is supported by the TIRF microscopy data, which indicate that AS160-4P blocks insulin-regulated recruitment of GLUT4 to within ∼250 nm of the cell surface. However, it is important to note that the results of the TIRF microscopy do not rule out the possibility that AS160 also has a role in the later steps of vesicle docking and fusion.
AS160-4P completely blocked GLUT4 translocation when measured by TIRF microscopy. The TIRF assay detects a threefold insulin-stimulated redistribution, which is considerably less than the translocation measured in the exposure of the HA-epitope assay. The reduced sensitivity of the TIRF assay may explain why we do not see a partial effect of AS160-4P on insulin-stimulated GLUT4 translocation.
How does AS160-4P regulate exocytosis? The current model for AS160 action is that phosphorylation by Akt inhibits its GAP activity, which would result in the activation of a Rab protein required for GLUT4 translocation to the cell surface (Sano et al., 2003). Numerous membrane trafficking processes are regulated by the actions of Rabs; therefore, insulin modulation of a RabGAP is an attractive mechanism for regulation of GLUT4 movement. However, without knowing the target Rab of AS160, further speculation on the precise mechanism regulated by AS160 is not warranted.
Finding, in direct measurements of internalization rate constants, that insulin regulates endocytosis of GLUT4 by a mechanism independent of its actions on GLUT4 exocytosis is important for understanding insulin's action on membrane traffic. These data demonstrate that the change in internalization is not an indirect effect of the increased exocytosis, as would be the case if the increased GLUT4 delivered to the cell surface in the presence of insulin saturated the uptake mechanism, and therefore that insulin is directly signaling to endocytosis mechanism. Which raises the question of how insulin signals to internalization? It has recently been reported that insulin signals to Rab5 by inhibiting its GTP loading and that insulin induces the increased binding of the motor protein dynein to microtubules, both of which are dependent on in a PI 3-kinase activity (Huang et al., 2001). These data support a role of PI 3-kinase in insulin signaling to GLUT4 endocytosis. In that study, it was also shown that microinjection of anti-Rab5 or anti-dynein antibodies decreased removal of GLUT4 from the cell surface after insulin withdrawal, which are consistent with Rab5 and dynein being involved in GLUT4 internalization, although additional studies are required to show that insulin inhibits GLUT4 endocytosis by its effects on Rab5 and dynein. It also has recently been reported that caveolae have a role in regulation of GLUT4 internalization, providing an additional possible mode for insulin signaling to endocytosis (Shigematsu et al., 2003). Future studies are required to determine the mechanism for insulin signaling to endocytosis.
There are significant data supporting a key role for Akt in insulin-stimulated GLUT4 translocation and glucose uptake. Here, we show that a constitutively active Akt1 quantitatively mimics the effects of insulin on GLUT4 trafficking, consistent with insulin activation of Akt being sufficient to account for the full translocation of GLUT4 (Kohn et al., 1996; Foran et al., 1999). However, it is possible that when Akt is constitutively active non-PI 3-kinase–dependent signaling pathways, which are normally required for insulin regulation of GLUT4 trafficking, are bypassed or activated.
In addition to insulin's pronounced affect on surface expression of GLUT4 and IRAP, insulin has a smaller stimulatory affect on the expression of a number of other membrane proteins. For example, insulin acutely increases the surface expression of the TR by approximately twofold. The relationship between insulin's small effect on TR (and other membrane proteins) and its large effect on GLUT4 and IRAP distributions is not understood. One possibility is that a small amount of TR “leaks” into the specialized GLUT4 pathway, and when cells are stimulated with insulin, this TR is mobilized to the surface along with GLUT4. Thus, the amount of TR redistributed is the amount of TR missorted to the specialized pathway. In this case, any treatment that effects GLUT4 movement would have a similar effect on the TR, albeit to a smaller degree. Our finding that myr-Akt1 mimics insulin's effect on GLUT4 but not on the TR argues against TR leaking into the specialized pathway. Our data are in agreement with the result of a previous study in which a mutant Akt rendered constitutively active by the substitution of aspartic acids for Ser473 and Thr308 was shown to induce redistribution of GLUT4 but not the TR (Foran et al., 1999). Consistent with insulin signaling to TR being independent of Akt action is our finding that AS160-4P did not inhibit insulin's effect on TR distribution. Together, these data support the proposal that insulin's effect on TR reflects a specific action of insulin signaling on general endocytic trafficking; however, at this time the physiological significance of this regulation is not known.
Acknowledgments
We thank Gus Lienhard and Morris Birnbaum for reagents and comments. We thank members of the McGraw laboratory for helpful discussions, Fred Maxfield for comments on the manuscript, and the Keck Foundation for the TIRF microscope. The work was supported by National Institutes of Health grants DK-52852 and DK-57689 to T.E.M.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–04–0333. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–04–0333.
References
- Bae, S.S., Cho, H., Mu, J., and Birnbaum, M.J. (2003). Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 278, 49530-49536. [DOI] [PubMed] [Google Scholar]
- Bryant, N., Govers, R., and James, D. (2002). Regulated transport of the glucose transporter GLUT4. Nat. Rev Mol. Cell. Biol. 3, p 267-277. [DOI] [PubMed] [Google Scholar]
- Czech, M.P., and Buxton, J.M. (1993). Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J. Biol. Chem. 268, 9187-9190. [PubMed] [Google Scholar]
- Deneka, M., Neeft, M., and van der Sluijs, P. (2003). Regulation of membrane transport by rab GTPases. Crit. Rev. Biochem. Mol. Biol. 38, 121-142. [DOI] [PubMed] [Google Scholar]
- Egawa, K., Nakashima, N., Sharma, P.M., Maegawa, H., Nagai, Y., Kashiwagi, A.,Kikkawa,R.,andOlefsky,J.M.(2000).Persistentactivationofphosphatidylinositol 3-kinase causes insulin resistance due to accelerated insulin-induced insulin receptor substrate-1 degradation in 3T3–L1 adipocytes. Endocrinology 141, 1930-1935. [DOI] [PubMed] [Google Scholar]
- Egawa, K., Sharma, P., Nakashima, N., Huang, Y., Huver, E., Boss, G., and Olefsky, J. (1999). Membrane-targeted phosphatidylinositol 3-kinase mimics insulin actions and induces a state of cellular insulin resistance. J. Biol. Chem. 274, p 14306-14314. [DOI] [PubMed] [Google Scholar]
- Foran, P.G., Fletcher, L.M., Oatey, P.B., Mohammed, N., Dolly, J.O., and Tavare, J.M. (1999). Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3–L1 adipocytes by a pathway involving SNAP-23,synaptobrevin-2,and/or cellubrevin. J. Biol. Chem. 274, 28087-28095. [DOI] [PubMed] [Google Scholar]
- Garza, L., and Birnbaum, M. (2000). Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J. Biol. Chem. 275, 2560-2567. [DOI] [PubMed] [Google Scholar]
- Guilherme, A., and Czech, M.P. (1998). Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J. Biol. Chem. 273, 33119-33122. [DOI] [PubMed] [Google Scholar]
- Hausdorff, S.F., Fingar, D.C., Morioka, K., Garza, L.A., Whiteman, E.L., Summers, S.A., and Birnbaum, M.J. (1999). Identification of wortmannin-sensitive targets in 3T3–L1 adipocytes. Dissociation of insulin-stimulated glucose uptake and glut4 translocation. J. Biol. Chem. 274, 24677-24684. [DOI] [PubMed] [Google Scholar]
- Hill, M.M., Clark, S.F., Tucker, D.F., Birnbaum, M.J., James, D.E., and Macaulay, S.L. (1999). A role for protein kinase Bbeta/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 19, 7771-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman, G.D., Lo Leggio, L., and Cushman, S.W. (1994). Insulin-stimulated GLUT4 glucose transporter recycling. A problem in membrane protein subcellular trafficking through multiple pools. J. Biol. Chem. 269, 17516-17524. [PubMed] [Google Scholar]
- Huang, J., Imamura, T., and Olefsky, J.M. (2001). Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc. Natl. Acad. Sci. USA 98, 13084-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakoff, S.J., Taha, C., Rose, E., Marcusohn, J., Klip, A., and Skolnik, E.Y. (1995). The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signaling pathways are required for insulin-stimulated glucose uptake. Proc. Natl. Acad. Sci. USA 92, 10247-10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhun, B.H., Rampal, A.L., Liu, H., Lachaal, M., and Jung, C.Y. (1992). Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. Evidence of constitutive GLUT4 recycling. J. Biol. Chem. 267, 17710-17715. [PubMed] [Google Scholar]
- Jiang, Z.Y., Zhou, Q.L., Coleman, K.A., Chouinard, M., Boese, Q., and Czech, M.P. (2003). Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 100, 7569-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, S., Sano, H., Liu, S.C., Asara, J.M., Lane, W.S., Garner, C.C., and Lienhard, G.E. (2002). A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 277, 22115-22118. [DOI] [PubMed] [Google Scholar]
- Karylowski, O., Zeigerer, A., Cohen, A., and McGraw, T.E. (2004). GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell 15, 870-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, A.D., Barthel, A., Kovacina, K.S., Boge, A., Wallach, B., Summers, S.A., Birnbaum, M.J., Scott, P.H., Lawrence, J.C., Jr., and Roth, R.A. (1998). Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 273, 11937-11943. [DOI] [PubMed] [Google Scholar]
- Kohn, A.D., Summers, S.A., Birnbaum, M.J., and Roth, R.A. (1996). Expression of a constitutively active Akt Ser/Thr kinase in 3T3–L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271, 31372-31378. [DOI] [PubMed] [Google Scholar]
- Lampson, M.A., Racz, A., Cushman, S.W., and McGraw, T.E. (2000). Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. J. Cell Sci. 113, 4065-4076. [DOI] [PubMed] [Google Scholar]
- Lampson, M.A., Schmoranzer, J., Zeigerer, A., Simon, S.M., and McGraw, T.E. (2001). Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles. Mol. Biol. Cell 12, 3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S., Millar, C.A., Lyttle, C.T., Meerloo, T., Marsh, B.J., Gould, G.W., and James, D.E. (2000). Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation. J. Cell Sci. 113, 3427-3438. [DOI] [PubMed] [Google Scholar]
- Moskowitz, H.S., Heuser, J., McGraw, T.E., and Ryan, T.A. (2003). Targeted chemical disruption of clathrin function in living cells. Mol. Biol. Cell 14, 4437-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki, V., Buxton, J., Chawla, A., Lifshitz, L., Fogarty, K., Carrington, W., Tuft, R., and Corvera, S. (2001). Insulin action on GLUT4 traffic visualized in single 3T3–l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 12, p 129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, S.A., Herbst, J.J., Keller, S.R., and Lienhard, G.E. (1997). Trafficking kinetics of the insulin-regulated membrane aminopeptidase in 3T3–L1 adipocytes. Biochem. Biophys. Res. Commun. 239, 247-251. [DOI] [PubMed] [Google Scholar]
- Saltiel, A., and Pessin, J. (2002). Insulin signaling pathways in time and space. Trends Cell Biol. 12, 65-71. [DOI] [PubMed] [Google Scholar]
- Sano, H., Kane, S., Sano, E., Miinea, C.P., Asara, J.M., Lane, W.S., Garner, C.W., and Lienhard, G.E. (2003). Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278, 14599-14602. [DOI] [PubMed] [Google Scholar]
- Semiz, S., Park, J., Nicoloro, S., Furcinitti, P., Zhang, C., Chawla, A., Leszyk, J., and Czech, M. (2003). Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 22, 2387-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu, S., Watson, R., Khan, A., and Pessin, J. (2003). The adipocyte plasma membrane caveolin functional/structural organization is necessary for the efficient endocytosis of GLUT4. J. Biol. Chem. 278, 10683-10690. [DOI] [PubMed] [Google Scholar]
- Slot, J.W., Geuze, H.J., Gigengack, S., Lienhard, G.E., and James, D.E. (1991). Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 113, 123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil, A., Lampson, M.A., Keller, S.R., and McGraw, T.E. (2000). Characterization of the insulin-regulated endocytic recycling mechanism in 3T3–L1 adipocytes using a novel reporter molecule. J. Biol. Chem. 275, 4787-4795. [DOI] [PubMed] [Google Scholar]
- Tengholm, A., and Meyer, T. (2002). A PI3-kinase signaling code for insulintriggered insertion of glucose transporters into the plasma membrane. Curr. Biol. 12, 1871-1876. [DOI] [PubMed] [Google Scholar]
- Wiese, R.J., Mastick, C.C., Lazar, D.F., and Saltiel, A.R. (1995). Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3–L1 adipocytes. J. Biol. Chem. 270, 3442-3446. [DOI] [PubMed] [Google Scholar]
- Yang, J., and Holman, G.D. (1993). Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3–L1 cells. J. Biol. Chem. 268, 4600-4603. [PubMed] [Google Scholar]
- Yeh, J.I., Verhey, K.J., and Birnbaum, M.J. (1995). Kinetic analysis of glucose transporter trafficking in fibroblasts and adipocytes. Biochemistry 34, 15523-15531. [DOI] [PubMed] [Google Scholar]
- Zeigerer, A., Lampson, M., Karylowski, O., Sabatini, D., Adesnik, M., Ren, M., and McGraw, T. (2002). GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell 13, 2421-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]