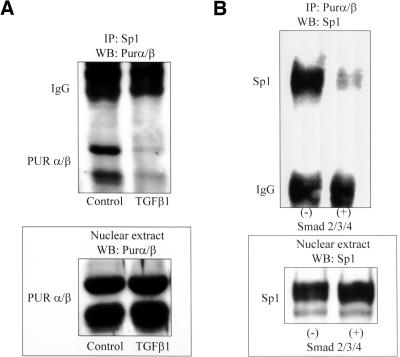
Figure 4.
Immunoprecipitation of a Sp1:Pur protein complex from fibroblasts. (A) Sp1 immunoprecipitate isolated from serum-free (control) AKR-2B fibroblast nuclear extracts contained prominent Purα and Purβ bands revealed by Western blot analysis by using a pan-specific anti-Pur antibody. Myofibroblast differentiation during a 6-h exposure to TGFβ1 was accompanied by diminished Sp1:Pur protein interaction. Exposure to TGFβ1 had no detectable effect on Pur protein isoform levels in nuclear extracts (bottom). (B) Pur protein immunoprecipitate isolated from COS7 fibroblasts transfected with empty expression plasmid (-) or equimolar mixtures of plasmids harboring cDNAs encoding Smad 2, 3, and 4 (+) were processed by Western blot by using a Sp1-specific antibody. Transfection with Smad expression plasmids did not effect net expression of Sp1 protein (bottom) but significantly impaired Sp1:Pur protein interaction (top).