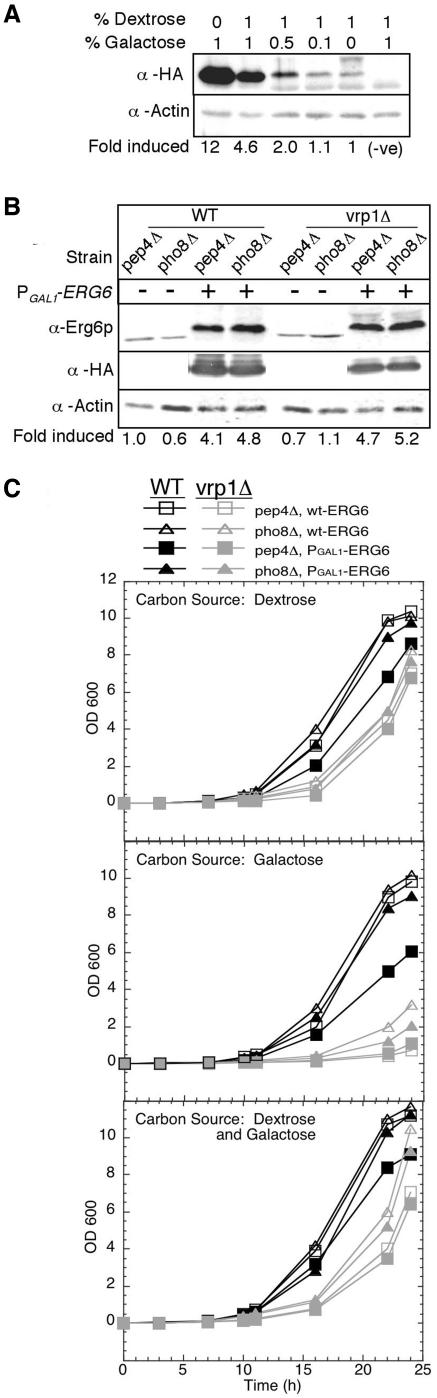
Figure 3.
Characterization of ERG6 overexpression strains. The galactose-regulated GAL1 promoter and three HA epitopes were introduced inframe at the 5′ end of the ERG6 gene. (A) Expression of PGAL1-HA-ERG6 was examined 24 h after inoculating 10 ml of YP media with varying dextrose:galactose ratios from a single YPD preculture of strain KTY3. Strain KTY1 was included as a negative control (no PGAL1-HA-ERG6 allele). Equivalent culture volumes were processed for immunoblot analysis with anti-HA and anti-actin as a load control. Bands were analyzed by densitometry and normalized to 1% dextrose to calculate fold induced. (B) Erg6p expression levels were examined in cultures grown in YP 1% dextrose/1% galactose to 1 OD600 for vacuole isolation. Culture equivalents of 0.25 OD600 units were processed from strains with PGAL1-HA-ERG6 induction, KTY3-4 (WT) and KTY7-8 (vrp1Δ), and from cultures without PGAL1-HA-ERG6, KTY1-2 (WT) and KTY5-6 (vrp1Δ). Immunoblot analysis was performed with both anti-Erg6p (gift from G. Daum) and anti-HA for direct comparison. Anti-Erg6p bands were analyzed by densitometry and normalized to wild-type pep4Δ (KTY1). (C) Growth curves were generated for wild-type (strains KTY1-4) and vrp1Δ mutants (strains KTY5-8) by inoculating 0.1 ml of 1 OD600 cultures into 50 ml of fresh YP media containing either 2% dextrose (top panel), 2% galactose (middle panel), or 1% dextrose/1% galactose (bottom panel).