Abstract
Background
Childhood maltreatment can trigger enduring changes in major stress response systems, particularly in the context of major depressive disorder (MDD). However, the relative impact of maltreatment versus MDD on hypothalamic-pituitary-adrenal axis and sympathetic-adrenal-medullary system stress reactivity is not well understood.
Method
This study examined salivary cortisol and alpha-amylase responses to the Trier Social Stress Test (TSST) in 26 maltreated (15 with current MDD) and 26 non-maltreated (17 with current MDD) women.
Results
Maltreated women showed greater anticipatory cortisol reactivity during the TSST protocol compared to non-maltreated women. Maltreated women also showed rapid deceleration in cortisol levels. Whereas non-maltreated women showed initial declines in alpha-amylase levels but rapidly increasing alpha-amylase levels during the TSST protocol, maltreated women did not exhibit changes in alpha-amylase levels during the TSST protocol. Contrary to expectation, MDD did not impact cortisol or alpha-amylase responses.
Limitations
The present study is limited by retrospective report of childhood maltreatment, cross-sectional design, and modest sample sizes.
Conclusions
These findings suggest that childhood maltreatment plays a greater role driving alterations in cortisol and alpha-amylase stress reactivity than MDD. Understanding the biological embedding of maltreatment is critical for elucidating mechanisms linking these experiences to risk for negative mental and physical health outcomes.
Keywords: maltreatment, women, cortisol, alpha-amylase, stress reactivity
Introduction
Maltreatment affects as many as 1 in 4 children in the United States and resulted in an estimated 3.6 million referrals for cases of suspected abuse or neglect and 1,580 childhood maltreatment-related deaths in 2014 (U.S. Department of Health and Human Services, 2016; Finkelhor et al., 2013). Exposure to childhood maltreatment (including physical, sexual or emotional abuse, physical or emotional neglect, and exposure to domestic violence) can produce enduring changes in stress response systems such as the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic-adrenal-medullary system (SAM), and can increase vulnerability to major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) (Heim et al., 2008; Pervanidou, 2008). Girls are more likely than boys to report maltreatment in general (U.S. Department of Health and Human Services, 2012), and sexual abuse in particular (Centers for Disease Control and Prevention, 2005). In retrospective studies, women are twice as likely as men to develop trauma-related conditions such as MDD in their lifetimes (Kessler et al., 1993).
Neurodevelopmental traumatology models emphasize the importance of both the developmental timing of trauma exposure as well as compensatory adaptations that occur over time. Unfortunately, time since maltreatment is confounded with age of maltreatment onset in most studies (Morris, Compas, & Garber, 2012). Regarding timing of trauma exposure, the impact of childhood trauma on physical and mental health outcomes is particularly devastating due to the adverse consequences of dysregulated stress response systems on brain development during critical vulnerability periods (De Bellis et al., 1999, 2011). For example, earlier onset of sexual abuse has been linked to smaller intracranial volume and greater PTSD symptom severity (De Bellis et al., 1999). Regarding time elapsed since trauma exposure, theoretical models describe an attenuation of HPA activity over time triggered by developmental alterations and compensatory adaptations of the HPA axis: children recently exposed to maltreatment exhibit increased diurnal cortisol secretion and reactivity to stress, but over time show a progressive downregulation of HPA (re)activity (De Bellis et al., 2011; Gunnar and Vazquez, 2001; Heim et al., 2008; Susman, 2006). Support for these models comes from a longitudinal study of youth with a history of sexual abuse (Trickett et al., 2010). In this longitudinal study of females from childhood through early adulthood, both abused and non-abused groups showed a linear increase in morning basal cortisol levels with development, but this trend leveled off in their early 20s. Abused females exhibited slower increases in cortisol levels over time (as they became older and time since abuse increased) compared to non-abused females.
It is challenging to interpret findings from studies of maltreated youth and adults examining HPA reactivity to psychosocial stress due to the host of genetic, developmental, personality, environmental and methodological factors that influence HPA function in general, and the stress response in particular (Allen et al., 2014). Nevertheless, one consistent theme in the literature has been the importance of accounting for psychiatric comorbidity such as MDD. Youth studies have reported elevated cortisol reactivity in boys and girls with mild-to-moderate depression but blunted cortisol reactivity in those with moderate-to-severe depression (Harkness et al., 2011) and elevated cortisol responses in depressed youth with early-life adversity (Rao et al., 2008). A third study that excluded youth who met diagnostic criteria for MDD reported blunted cortisol reactivity in adolescent girls regardless of depressive or PTSD symptoms (MacMillan et al., 2009). Adult studies have generally reported enhanced HPA reactivity to stress in maltreated individuals with MDD (Heim et al., 2000, 2002), PTSD (Bremner et al., 2003; Elzinga et al., 2003) or social anxiety disorder (Elzinga et al., 2010) compared to non-maltreated controls. In contrast, studies examining HPA responses to stress in maltreated adults without PTSD or MDD have generally reported blunted cortisol reactivity to stress (Carpenter et al., 2007, 2011; Elzinga et al., 2008; Voellmin et al., 2015 see also DeSantis et al., 2011; Luecken and Appelhans, 2006).
The SAM responds within seconds of stressor exposure by releasing norepinephrine and epinephrine from the adrenal medulla, which triggers a number of peripherally measurable physiological changes. Salivary alpha-amylase levels increase in response to psychosocial stressors, correlate positively with cardiovascular responses, and can be used as non-invasive indicators of SAM activation (Nater et al., 2006). Increased SAM activity following childhood trauma exposure has been proposed as a risk factor for developing PTSD (Pervanidou, 2008). However, it remains unclear whether individuals with a history of childhood maltreatment exhibit enduring changes in SAM reactivity similar to those observed for the HPA axis, and if these alterations differ according to the presence of MDD. The present study addressed this critical gap in the literature by simultaneously examining HPA and SAM reactivity to psychosocial stress in women exposed to childhood maltreatment, some of whom developed MDD while others were free from psychopathology.
Although enhanced SAM reactivity to stress appears to characterize trauma-exposed individuals with and without PTSD (for reviews see Morris and Rao, 2013; Pole, 2007), the impact of childhood maltreatment on SAM responses to psychosocial stressors has not been well characterized. One study in youth found an association between maltreatment history and cardiovascular parameters thought to reflect a threat (or withdrawal) response: blunted cardiac output reactivity and greater peripheral resistance (McLaughlin et al., 2014). Adult studies examining cardiovascular responses to stress tasks have reported elevated blood pressure in men and women with early parental loss (Luecken,1998), as well as elevated heart rate in women with PTSD due to childhood sexual abuse (Orr et al., 1998) or women with MDD in the context of childhood sexual or physical abuse (Heim et al., 2000) compared to non-maltreated controls. In contrast, a study of healthy women found more attenuated heart rate reactivity to a stressor among women with more adverse childhood experiences (Voellmin et al., 2015). Two studies examining relations between HPA and SAM reactivity to stress found an asymmetry between salivary cortisol and alpha-amylase responses in maltreated youth (Gordis et al., 2008) or higher ratios of alpha-amylase to cortisol responses in adults with more early-life adversity (Ali and Pruessner, 2012). Unfortunately, neither of these studies were able to parse the relative influence of maltreatment and trauma-related psychopathology.
The purpose of the present study was to examine the unique effects of childhood maltreatment and current MDD on salivary cortisol and alpha-amylase responses to a psychosocial stressor in women. We hypothesized that cortisol responses would be higher in maltreated women with current MDD and blunted in maltreated women without current MDD compared to non-maltreated women. We further hypothesized that alpha-amylase responses would be greater in maltreated women with current MDD compared to those without past maltreatment.
Methods
Participants
The participants were recruited through advertisements in local newspapers and universities as part of a larger programmatic study on depression. Participants for the present study were selected from this larger sample if they were 18 years or older, had completed a psychosocial stress task, and met further inclusion/exclusion criteria described below. The sample included 26 women with a history of maltreatment before the age of 10 (15 with current MDD) and 26 women with no history of maltreatment (17 with current MDD). Depressed women with a lifetime history of mania, hypomania, schizophrenia, schizoaffective disorder, or autism, or with a family history of bipolar disorder, were excluded from the study. All participants were medically healthy and free from psychotropic medication (for a minimum of 8 weeks but most of them were psychotropic-naive), other medications that can impact HPA and SAM activity (with the exception of oral contraceptives), and alcohol or illicit drug use, as determined by physical examination, laboratory investigations, and urine drug screens. All participants provided written informed consent and all procedures were approved by the institutional review board.
Measures
Maltreatment
The Childhood Adversity Interview (CAI; Dienes et al., 2006; Rao et al., 2008; Raposa et al., 2014) is a semi-structured interview that was administered by trained raters. Seven subtypes of childhood adversity occurring before age 10 were assessed: separation/loss; life-threatening illness or injury; physical neglect; emotional abuse; physical abuse or assault; witnessing domestic violence; and sexual abuse or assault. Summary scores for each subtype were tabulated using ratings on a 5-point scale (1 = none, 5 = most severe). The presence of maltreatment (dummy variable: 1 = present; 0 = absent) was determined by a score of three or greater on at least one of the following subscales: physical abuse/assault, sexual abuse/assault, and witnessing domestic violence. The CAI has demonstrated good inter-rater and test-retest reliability (Hammen et al., 2000). Inter-rater reliability scores in this sample computed for a randomly selected 25% of CAI interviews were adequate (κ’s ≥ 0.85).
Depression
The diagnosis of MDD and other psychiatric disorders was based on the Structured Clinical Interview for DSM-IV (SCID-I; First et al., 1997). A randomly selected 25% of assessments were independently coded for inter-rater reliability (κ’s ≥ 0.85). Self-reported depressive symptoms were assessed with the Beck Depression Inventory (BDI; Beck et al., 1961).
Psychosocial Stressor
A standardized psychosocial stress protocol – the Trier Social Stress Test (TSST) - was used to induce HPA and SAM reactivity (Buske-Kirschbaum, et al., 2001 Kirschbaum et al., 1993) that involved a 2-hour pre-stress period, a 5-minute public-speaking task (following a 5-minute preparation period) followed by a 5-minute mental arithmetic task performed in front of an audience, and a 60-minute post-stress period. Participants were informed during the consenting process that they would be performing challenging tasks but these tasks were not described until immediately prior to the 5-minute preparation period. Pre-stress saliva samples were collected at 30-minute intervals for 2 hours prior to the stress task, with the final sample provided immediately prior to the stress tasks (five samples). Post-stress saliva samples were collected immediately after the tasks and at 10-minute intervals for 60 minutes (seven samples). Participants arrived at the laboratory at 4:30 pm and the TSST protocol (with baseline pre-stress saliva sampling) began at 5:00pm. Following the TSST protocol, participants used a visual analogue scale to indicate how stressful they experienced the TSST (1 = not at all; 3 = a little; 5 = very stressful).
Assays
Salivary cortisol levels were determined in duplicate using a commercially available enzyme immunoassay kit (Enzyme-Linked ImmunoSorbent Assay, ALPCO diagnostics, Salem, NH). Salivary alpha-amylase was determined in duplicate by kinetic assay (Salimetrics LLC, State College, PA). The intra- and inter-assay coefficients of variation for the assays were less than 10%. Samples from the same subject were analyzed in the same assay.
Socioeconomic status (SES)
SES was calculated using the Hollingshead four-factor index (Hollingshead, 1975).
Data Analytic Plan
Cortisol and alpha-amylase data were log-transformed to reduce skewness. Pre-stress hormone/enzyme levels were computed as the mean of the fourth and fifth baseline samples, and the first three baseline samples were considered as acclimation to the laboratory and not included in the analysis. To examine within- and between-individual changes simultaneously, we specified a multilevel model (MLM) using Hierarchical Linear Models (HLM v. 6; Raudenbush et al., 2004) consisting of a within-person (i.e., level 1) sub-model describing cortisol and alpha-amylase responses to each TSST and between-person (i.e., level 2) sub-model describing how cortisol/alpha-amylase responses to each TSST varied between maltreated/non-maltreated and current MDD/non-MDD groups. These MLMs used restricted maximum likelihood estimation. Standard assumptions regarding normality and homoscedasticity of level 1 and 2 residuals were met (Bryk and Raudenbush, 1992; Singer and Willett, 2003). Dummy codes were used to indicate the presence of maltreatment and/or current MDD (1 = present; 0 = absent). We examined quadratic change trajectories for cortisol/alpha-amylase levels in response to the TSST.
The 2-level MLM model was as follows:
- Level 1 Model:
- Level 2 Model:
In the quadratic change model presented above, π0 represents the cortisol/alpha-amylase trajectory’s intercept at the start of the TSST, π1 represents the instantaneous rate of change in cortisol/alpha-amylase levels immediately prior to the TSST (i.e., anticipatory reactivity), and π2 represents how this rate of change increases or decreases over the course of the TSST (i.e., curvature) (Singer and Willett, 2003). For example, an individual may exhibit elevated cortisol/alpha-amylase anticipatory reactivity (i.e., a positive value for π1), but this rate of increase could slow over time such that cortisol/alpha-amylase levels reach a peak and eventually decline (i.e., a negative value for π2). MLM was used to test the cross-level interaction of maltreatment X MDD X saliva sampling time predicting cortisol/alpha-amylase responses to the TSST as well as the 2-way interactions of maltreatment and MDD status with saliva sampling time. Significant interactions were probed and simple slopes were calculated using Preacher and colleagues’ online calculator (Preacher et al., 2006). There were no biologically implausible values for cortisol or alpha-amylase levels, nor were there outliers (defined as 3 or more standard deviations from the mean). Three participants were missing one or more cortisol data points and four participants were missing one or more alpha-amylase data points. Missing data at Level 1 was handled using maximum-likelihood estimation.
Results
Demographic and clinical characteristics of maltreated and non-maltreated women are presented in Table 1. Preliminary analyses revealed that the maltreated and non-maltreated groups did not differ significantly in racial or ethnic composition, age, percentage of individuals with a current diagnosis of MDD, or in self-reported depressive symptoms. Comorbid anxiety disorders in the sample included generalized anxiety disorder (n = 2), panic disorder (n = 2) and social phobia (n = 2). Maltreated women reported higher overall levels of childhood adversity and significantly higher scores on all adversity subtypes except life-threatening illness/injury and physical neglect. Paired t-tests indicated that cortisol levels increased significantly from pre-TSST (mean = 0.09, SD = 0.17) to peak post-TSST (mean = 0.19, SD = 0.33) in non-maltreated women (t(25) = 2.11, p = .045) and showed a non-significant trend for an increase from pre-TSST (mean = 0.05, SD = 0.12) to peak post-TSST (mean = 0.22, SD = 0.54) in maltreated women (t(25) = 1.94, p = .064). Paired t-tests indicated that alpha-amylase levels increased significantly from pre-TSST (mean = 69.01, SD = 46.41) to peak post-TSST (mean = 106.55, SD = 64.69) in non-maltreated women (t(25) = 4.02, p = .001) and also increased significantly from pre-TSST (mean = 88.81, SD = 82.93) to peak post-TSST (mean = 124.07, SD = 95.07) in maltreated women (t(25) = 4.64, p < .001). Cortisol and alpha-amylase output in response to the TSST, indexed by area under the curve with respect to ground, was positively correlated for both maltreated (r(24) = .63, p = .001) and non-maltreated women (r(24) = .53, p = .007). Most participants rated the TSST as either “stressful” or “very stressful,” as evident in a mean visual analogue score of 4.4 (SD = 0.8).
Table 1.
Descriptive Data for Maltreated and Non-Maltreated Women
| Maltreated (n = 26) |
Non-Maltreated (n = 26) |
Maltreated vs. Non- Maltreated |
|
|---|---|---|---|
| N (%) | N (%) | X2 | |
| Race | 3.87 | ||
| Caucasian | 7 (27) | 13 (50) | |
| African American | 12 (46) | 8 (31) | |
| Asian | 2 (8) | 2 (8) | |
| Multiracial | 5 (19) | 3 (11) | |
| Ethnicity | |||
| Hispanic | 4 (15) | 3(11) | 0.17a |
| Non-Hispanic | 22 (85) | 3(89) | |
| MDD | 15 (58) | 17(65) | 0.33 |
| M (SD) | M (SD) | t | |
| Age | 26.2 (9.5) | 28.7 (9.4) | 0.98 |
| Socioeconomic status | 35.2 (11.4) | 41.9 (13.2) | 1.94 |
| BDI score | 20.5 (11.7) | 17.8 (12.3) | 0.77 |
| Maltreatment onset age | 5.3 (2.8) | -- | |
| CAI scores | |||
| Total adversity | 15.4 (3.7) | 10.6 (4.5) | 4.02*** |
| Separation | 2.1 (1.0) | 1.4 (0.7) | 3.02** |
| Illness/injury | 2.5 (1.5) | 1.9 (1.5) | 1.27 |
| Physical neglect | 1.6 (1.0) | 1.3 (0.6) | 1.05 |
| Emotional abuse | 2.1 (1.2) | 1.4 (0.8) | 2.48* |
| Physical assault/abuse | 2.5 (1.0) | 1.8 (1.0) | 2.34* |
| Sexual assault/abuse | 2.1 (1.5) | 1.4 (0.9) | 2.22* |
| Witnessing violence | 2.4 (1.2) | 1.4 (0.9) | 3.30** |
Note: MDD = major depressive disorder; BDI = Beck Depression Inventory; CAI = Childhood Adversity Interview.
Chi-square test using continuity correction.
p < .001;
p < .01;
p < .05.
Multilevel Models Predicting Within-Individual Change in Cortisol Responses
Preliminary analyses without substantive predictors confirmed that the TSST successfully induced cortisol responses in the full sample, as evident in both anticipatory cortisol reactivity (b = .008, SE = .003, p < .001) and quadratic change (b = −.0001, SE = .00004, p < .001). The maltreatment X MDD X time interaction (i.e., anticipatory reactivity) was significant (b = −.017, SE = .009, p = .046). Simple slope analyses revealed stronger anticipatory cortisol reactivity for maltreated women without current MDD (b = .020, SE = .006, p < .001) compared to those with current MDD (b = .014, SE = .005, p = .005). In contrast, non-maltreated women with (b = .003, SE = .005, p = .513) and without current MDD (b = −.008, SE = .005, p = .110) did not exhibit anticipatory cortisol reactivity.
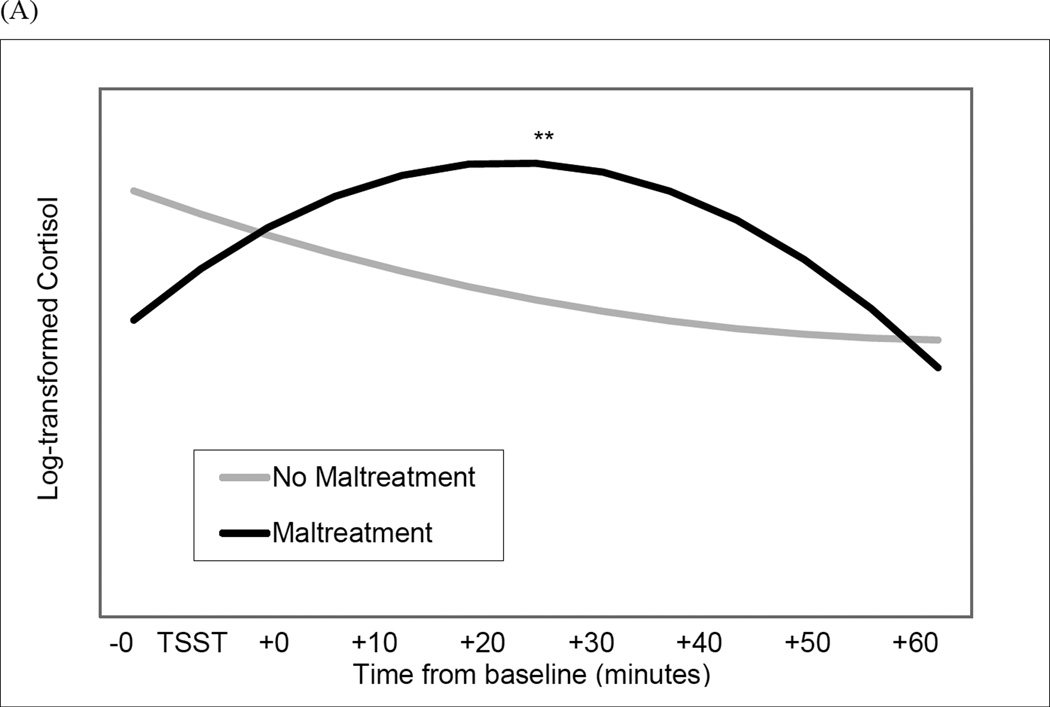
The maltreatment X MDD status X time2 interaction (i.e., quadratic change) was not significant (b = .0002, SE = .0001, p = .075). Therefore, we examined the maltreatment X time2 and MDD X time2 interactions. The interaction of maltreatment and quadratic change was significant (b = −.0003, SE = .0001, p < .001) (Figure 1, Panel A). Simple slope analyses revealed deceleration in cortisol levels during the TSST (i.e., slowing rate of change over time) for maltreated women (b = −.0002, SE = .00001, p < .001), consistent with a robust initial cortisol response and rapid cortisol recovery. In contrast, non-maltreated women did not exhibit quadratic change in cortisol levels during the TSST (b = .0001, SE = .0001, p = .430). Current MDD was not associated with quadratic change in cortisol levels (b = −.0001, SE = .0001, p = .136).
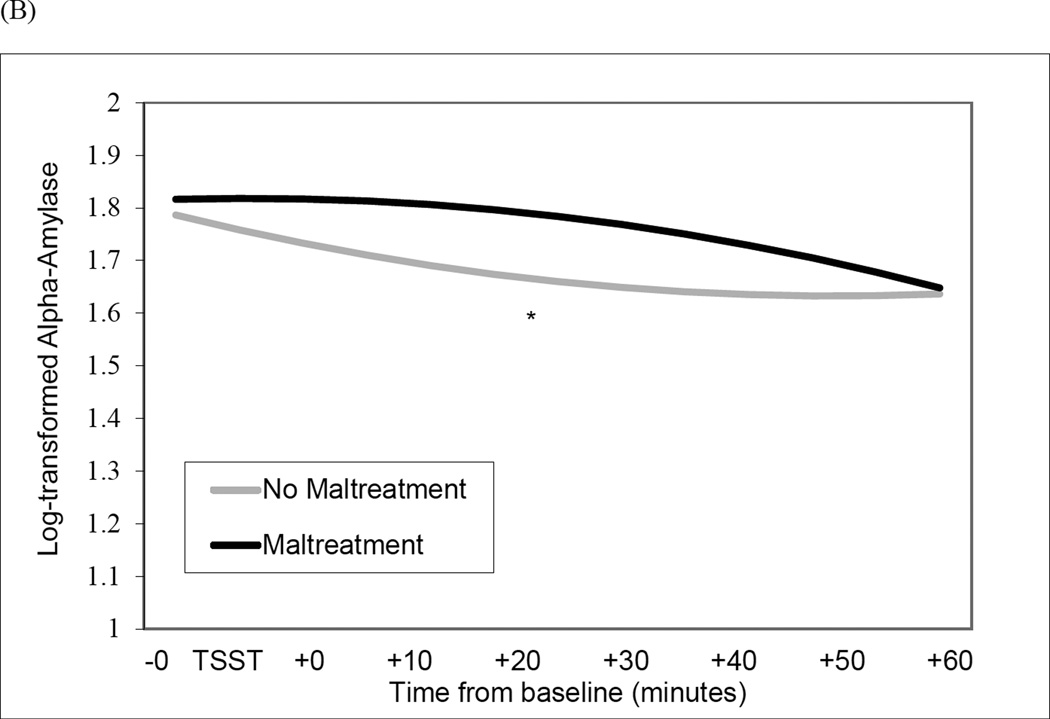
Figure 1.
Interaction of Maltreatment and sampling time predicting quadratic change in cortisol levels (Panel A) and alpha-amylase levels (Panel B) during the Trier Social Stress Test (TSST) protocol. **p < .001; *p < .05.
Multilevel Models Predicting Within-Individual Change in Alpha-Amylase Responses
Preliminary analyses in the full sample without substantive predictors revealed that the TSST-induced changes in anticipatory alpha-amylase reactivity (b = −.003, SE = .001, p = .015) but not quadratic change (b = .00003, SE = .00002, p = .112). The maltreatment X MDD X time (b = −.007, SE = .008, p = .363) and maltreatment X MDD X time2 (b = .0001, SE = .0001, p = .261) interactions were not significant. Therefore, we tested the two-way interactions of maltreatment and MDD with anticipatory reactivity and quadratic change.
The maltreatment X time (i.e., anticipatory reactivity) was significant (b = .006, SE = .003, p = .016). Simple slope analyses revealed that alpha-amylase levels were declining immediately prior to the TSST for non-maltreated women (b = −.005, SE = .002, p = .013). In contrast, maltreated women did not exhibit alpha-amylase anticipatory reactivity (b = .001, SE = .001, p = .115).
The maltreatment X time2 (i.e., quadratic change) was also significant (b = −.0001, SE = .00004, p = .022) (Figure 1, Panel B). Simple slope analyses revealed acceleration in alpha-amylase levels during the TSST (i.e., increasing rate of change over time) for non-maltreated women (b = .0001, SE = .00001, p = .040), evident in declining levels immediately prior to the TSST but rapidly increasing alpha-amylase levels during the recovery phase. In contrast, maltreated women did not exhibit quadratic change in alpha-amylase levels during the TSST (b = −.0001, SE = .0001, p = .094). Current MDD was not significantly associated with linear (B = − .002, SE = .003, p = .458) or quadratic change in alpha-amylase levels (B = .00005, SE = .00004, p = .180).
Discussion
Depressed individuals with a history of childhood maltreatment are more likely to have recurrences and less likely to respond to treatment than those without exposure to maltreatment (Nanni et al., 2012). Depressed women with childhood abuse also show greater HPA reactivity than non-abused depressed women (Heim et al., 2000). These findings suggest that MDD in the context of early-life adversity may represent a distinct subtype characterized by enhanced stress sensitivity (Heim et al., 2004). The present study sought to parse the relative influence of maltreatment and current MDD by simultaneously assessing both HPA and SAM responses to a psychosocial stressor in women. Maltreated women exhibited enhanced stress sensitivity evident in greater anticipatory cortisol reactivity than women without exposure to maltreatment. Maltreated women also showed a pattern of rapid cortisol recovery. Contrary to expectations, maltreated women exhibited no changes in alpha-amylase levels (i.e., neither anticipatory reactivity nor quadratic change during the TSST) whereas non-maltreated women showed an anticipatory decline in alpha-amylase levels followed by an acceleration during the TSST. Current MDD moderated the relation between maltreatment and anticipatory cortisol reactivity but did not moderate relations between maltreatment and quadratic changes in cortisol or alpha-amylase levels.
How can the present findings showing robust cortisol responses regardless of current MDD be reconciled with other psychosocial challenge studies showing blunted HPA reactivity in maltreated adults without psychopathology (e.g., Carpenter et al., 2007; Elzinga et al., 2008; Voellmin et al., 2015)? One explanation is that the direction and strength of this relationship depends on recent stress exposure and age of the samples. Among adolescents with greater exposure to early-life adversity, cortisol responses were significantly higher in those currently experiencing chronic stress (Rao et al., 2008). In addition, the combination of childhood abuse and trauma in adulthood was associated with heightened HPA reactivity in women (Heim et al., 2002). Hence, it may be chronic stress – rather than current psychopathology per se – that amplifies HPA reactivity in adults who experienced childhood maltreatment (Bremner et al., 2003; Elzinga et al., 2003, 2010; Heim et al., 2000, 2002). A second possibility is that the negative impact of maltreatment could be mitigated or exacerbated by parenting practices. For example, among children with greater trauma-exposure, cortisol reactivity was lower in those with less harsh and more supportive parenting and elevated in those with more harsh and less supportive parenting (Jaffee et al., 2015). Similarly, cortisol responses to stress were elevated among abused adults who experienced early parental loss but not among abused adults with intact families (Luecken and Appelhans, 2006). Finally, the pattern of robust cortisol increases and rapid cortisol recovery may indicate greater resilience in the present sample compared to other studies of maltreated women. Consistent with this perspective, exogenous cortisol administration has been shown to protect against negative emotional responses to acute stressors (Het and Wolf, 2007). On the other hand, blunted cortisol reactivity may reflect maladaptive coping strategies (e.g., disengagement or avoidance) and hypocortisolism is a risk factor for negative health outcomes (Susman, 2006; Trickett et al., 2014).
Alpha-amylase levels in women with maltreatment history appeared flat and unresponsive to the TSST, which contrasted with initial decreases and subsequent acceleration of alpha-amylase levels during the TSST in non-maltreated women. This result is surprising given evidence of elevated SAM responses to stressors in traumatized adults with or without PTSD (Morris and Rao, 2013; Pole, 2007) and those exposed to childhood maltreatment (Heim et al., 2000; Luecken, 1998; Orr et al., 1998). One explanation is that salivary alpha-amylase should not be considered a pure measure of SAM activity, but rather is a product of both SAM and parasympathetic nervous system (PNS) activation (Proctor and Carpenter, 2007). This could explain the generally small correlations between alpha-amylase and measures of SAM activity reported by previous studies (e.g., El-Sheikh et al., 2008; Nater et al., 2006; Thoma et al., 2012).
Alpha-amylase release is influenced by SAM activity (Proctor and Carpenter, 2007) and typically increases following stress exposure (Takai et al., 2004). PNS activity diminishes during acute stress (Berntson et al., 2007) but may produce ‘augmented secretion’ of alpha-amylase when PNS activity rebounds immediately following stressor offset (Proctor and Carpenter, 2007). Some researchers have speculated that the transient increase in alpha-amylase levels immediately post-stressor may “reflect a relief response, rather than a stress response” (Nagy et al., 2015, p. 117). Such a response – whether relief or stress-related – was observed in the acceleration of alpha-amylase levels following the TSST for non-maltreated women. In contrast, the absence of an alpha-amylase response in maltreated women might reflect PNS dysregulation - or a failure to co-activate SAM and PNS systems (Rottenberg et al., 2003). Altered PNS activity, as evident in lower heart rate variability, has been found in individuals at risk for PTSD (Minassian et al., 2015) but has not, to our knowledge, been documented in adults with childhood maltreatment. Given the implications of different SAM by PNS interaction patterns for the development of psychopathology in children exposed to early-life adversity (El-Sheikh et al., 2009), future studies should assess “pure” indicators of PNS and SAM stress reactivity in addition to salivary alpha-amylase.
Interactions between SAM and HPA responses to stress in maltreated individuals are also not well understood (Granger et al., 2007). A study conducted in youth found a significant association between cortisol and alpha-amylase reactivity in those without maltreatment but a lack of correspondence between these biomarkers in maltreated youth (Gordis et al., 2008). In contrast, the present study found strong positive correlations between alpha-amylase and cortisol stress responses regardless of maltreatment history. Results revealed more robust cortisol responses in maltreated compared to non-maltreated women, which is consistent with sensitization of the HPA-axis (Kendall-Tackett, 2000; Yehuda et al., 1996). However, maltreated women did not exhibit the acceleration in alpha-amylase levels following the TSST that was observed in non-maltreated women. It is possible, given theoretical models and empirical evidence highlighting the role of glucocorticoids in suppressing SAM activity (Irwin et al., 1986; Munck et al., 1984; Sapolsky et al., 2000), that rapid increases in cortisol levels early in the TSST (i.e., greater anticipatory reactivity) inhibited alpha-amylase secretion in women with maltreatment.
Limitations of the present study highlight directions for future research. First, childhood maltreatment was determined by a semi-structured interview, which provides the opportunity for the interviewer to establish rapport with participants and obtain more detailed information about traumatic experiences than many self-report measures but nevertheless is reliant on retrospective report and subject to under-reporting. Second, neglect was not included as a child maltreatment subtype, which limits generalization of the present findings to the broader literature on child maltreatment. Third, the present study was conducted in women only, which limits generalizability and precludes examination of potential sex differences. Fourth, although the maltreatment X MDD status X time interaction predicting anticipatory cortisol reactivity was significant, the modest sample size may have limited power to test the joint influence of maltreatment and current MDD on quadratic changes in cortisol and alpha-amylase levels. Fifth, the study design precludes us from distinguishing stress reactivity patterns as either trait or scar markers of maltreatment (Adam et al., 2008). Sixth, although significant increases in alpha-amylase from baseline to peak levels were detected using paired t-tests in both maltreated and non-maltreated women, multilevel models failed to detect significant within-individual changes in alpha-amylase levels in maltreated women. Despite these limitations, the present study possessed a number of strengths: rigorous pre- and post-TSST sampling protocol, sophisticated data-analytic strategy, and careful psychiatric and medical screening.
The present study demonstrated patterns of cortisol and alpha-amylase responses to psychosocial stress that differentiated women with and without a history of childhood maltreatment. These findings have the potential to inform theoretical models of stress sensitization (Espejo et al., 2006) by highlighting alterations of HPA and SAM stress reactivity associated with maltreatment that could increase vulnerability to develop psychopathology when confronted with stressful life events. Understanding the biological embedding of early-life adversities is critical for elucidating the mechanisms linking these experiences to long-term risk for negative mental and physical health outcomes (McEwen, 2013).
Highlights.
Childhood maltreatment and depression trigger enduring changes in HPA function.
It is unknown whether maltreated adults exhibit altered sympathetic reactivity.
Stress reactivity was examined in 52 women with and without prior maltreatment.
Prior maltreatment predicted greater cortisol stress reactivity.
Maltreated women did not exhibit significant alpha-amylase stress reactivity.
Acknowledgments
This research was funded in part by grants from the National Institute of Health (R01 DA014037, R01 DA015131, R01 DA017804, R01 DA017805, R01 MH062464, R01 MH068391, and K01 MH101403), by the Sarah M. and Charles E. Seay Chair in Child Psychiatry at UT Southwestern Medical Center (Uma Rao), and by the Betsey R. Bush Endowed Professorship in Behavioral Health at the University of Tennessee (Uma Rao). These funding agencies had no further role in the study design, data collection, analysis or interpretation of data, writing of the report, or the decision to submit the manuscript for publication. The authors would like to thank all the volunteers who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic-pituitary-adrenal axis measures into preventive interventions for adolescent depression: Are we there yet? Dev. Psychol. 2008;20:975–1001. doi: 10.1017/S0954579408000461. [DOI] [PubMed] [Google Scholar]
- Ali N, Pruessner JC. The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiol. Behav. 2012;106:65–72. doi: 10.1016/j.physbeh.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;40:1228–1231. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Lozano D. Cacioppo JT, Tassinary LG, Bernston GG, editors. Cardiovascular psychophysiology. Handbook of Psychophysiology, third ed. 2007:182–210. Cambridge University Press. [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer S, Afzal N, McGlashan T, Elzinga B, Anderson GM, Heninger G, Southwick SM, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks: Sage; 1992. [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom. Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology. 2011;214:367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2005. Adverse childhood experiences study: Data and statistics. [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology part I: Biological stress systems. Biol. Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Spratt EG, Hooper SR. Neurodevelopmental biology associated with childhood sexual abuse. J. Child Sex. Abus. 2011;20:548–587. doi: 10.1080/10538712.2011.607753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis SM, Baker NL, Back SE, Spratt E, Ciolino JD, Moran-Santa Maria M, Dipankar B, Brady KT. Gender differences in the effect of early life trauma on hypothalamic-pituitary-adrenal axis functioning. Depress. Anxiety. 2011;28:383–392. doi: 10.1002/da.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes KA, Hammen C, Henry RM, Cohen AN, Daley SE. The stress sensitization hypothesis: Understanding the course of bipolar disorder. J. Affect. Disord. 2006;95:43–49. doi: 10.1016/j.jad.2006.04.009. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. J. Abnorm. Child Psychol. 2008;36:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children’s externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monogr. Soc. Res. Child. 2009;74:1–18. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: A study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Spinhoven P, Berretty E, de Jong P, Roelofs K. The role of childhood abuse in HPA-axis reactivity in social anxiety disorder: A pilot study. Biol. Psychol. 2010;83:1–6. doi: 10.1016/j.biopsycho.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Espejo EP, Hammen CL, Connolly NP, Brennan PA, Najman JM, Bor W. Stress sensitization and adolescent depressive severity as a function of childhood adversity: A link to anxiety disorders. J. Abnorm. Child Psychol. 2006;35:287–299. doi: 10.1007/s10802-006-9090-3. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Turner HA, Shattuck A, Hamby SL. Violence, crime, and abuse exposure in a national sample of children and youth: An update. JAMA Pediatr. 2013;167:614–621. doi: 10.1001/jamapediatrics.2013.42. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) - Clinician Version. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm. Behav. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research: Recent developments and applications. AnnN.YAcad. Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. J. Consult. Clin. Psych. 2000;68:782–787. [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Front. Behav. Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: A multiple regression analysis. Depress. Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Het S, Wolf OT. Mood changes in response to psychosocial stress in healthy young women: Effects of pretreatment with cortisol. Behav. Neurosci. 2007;121:11–20. doi: 10.1037/0735-7044.121.1.11. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Irwin J, Ahluwalia P, Anisman H. Sensitization of norepinephrine activity following acute and chronic footshock. Brain Res. 1986;379:98–103. doi: 10.1016/0006-8993(86)90260-x. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, McFarquhar T, Stevens S, Ouellet-Morin I, Melhuish E, Belsky J. Interactive effects of early and recent exposure to stressful contexts on cortisol reactivity in middle childhood. J. Child Psychol. Psychiatry. 2015;56:138–146. doi: 10.1111/jcpp.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett KA. Physiological correlates of childhood abuse: Chronic hyperarousal in PTSD, depression, and irritable bowel syndrome. Child Abuse Negl. 2000;24:799–810. doi: 10.1016/s0145-2134(00)00136-8. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity, and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Luecken L. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosom. Med. 1998;60:765–772. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Appelhans BM. Early parental loss and salivary cortisol in young adulthood: The moderating role of family environment. Dev. Psychopathol. 2006;18:295–308. doi: 10.1017/S0954579406060160. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Muelen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the youth mood project. Biol. Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The brain on stress: Toward an integrative approach to brain, body, and behavior. Perspect. Psychol. Sci. 2013;8:673–675. doi: 10.1177/1745691613506907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity: Identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosom. Med. 2014;76:538–546. doi: 10.1097/PSY.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB. Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 2015;72:979–986. doi: 10.1001/jamapsychiatry.2015.0922. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U. Psychobiology of PTSD in the acute aftermath of trauma: Integrating research on coping, HPA function and sympathetic nervous system activity. Asian J. Psychiatr. 2013;6:3–21. doi: 10.1016/j.ajp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nagy T, van Lien R, Willemsen G, Proctor G, Efting M, Fülöp M, Bárdos G, Veerman ECI, Bosch JA. A fluid response: Alpha-amylase reactions to acute laboratory stress are related to sample timing and saliva flow rate. Biol. Psychol. 2015;109:111–119. doi: 10.1016/j.biopsycho.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity – associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Berry NJ, Ahern CE, Pitman RK. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. J. Consult. Clin. Psych. 1998;66:906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. J. Neuroendocrinology. 2008;20:632–638. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychol. Bull. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J. Educ. Behav. Stat. 2006;31:437–448. [Google Scholar]
- Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol. Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa EB, Hammen CL, Brennan PA, O'Callaghan F, Najman JM. Early adversity and health outcomes in young adulthood: the role of ongoing stress. Health Psychol. 2014;33:410–418. doi: 10.1037/a0032752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows. Lincolnwood, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Ritchie KL, Holden GW. Parenting stress in low income battered and community women: Effects on parenting behavior. Early Educ. Dev. 1998;9:97–112. [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40:1–6. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neurosci. Biobehav. Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004;49:963–968. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol. Psychol. 2012;91:342–248. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Gordis E, Peckins MK, Susman EJ. Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment. 2014;19:27–37. doi: 10.1177/1077559513520466. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psychopathol. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. Child maltreatment 2014. 2016 Available from http://www.acf.hhs.gov/programs/cb/research-data-technology/statistics-research/child-maltreatment.
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biol. Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]