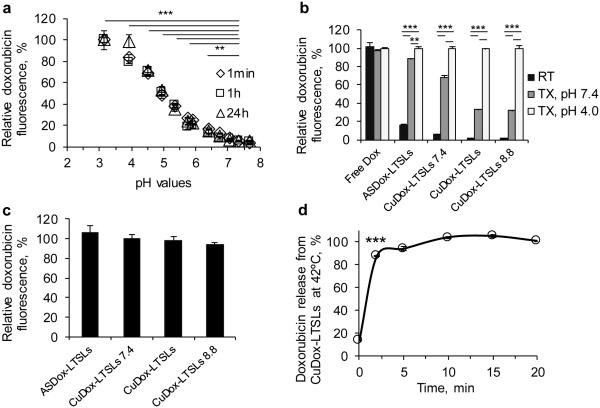
Figure 1. Stability of CuDox complex at higher pH during loading and disruption by reduced pH after release.
CuDox liposomes were prepared with an intraliposomal pH of 7.4, 8.4, or 8.8 (CuDox-LTSLs7.4, CuDox-LTSLs, and CuDox-LTSLs8.8, respectively) and were compared with ASDox-LTSLs and free Dox. a) Dox fluorescence after disassociation of CuDox complex following exposure to BSA solutions with varied pH at 37°C. In a), CuDox complex was created by adding Dox to Cu/TEA solution (pH 7.4) in the absence of liposomes. Fluorescence intensity is presented as percent of the maximum fluorescence intensity of free Dox obtained at pH 3. b) Dox fluorescence after 30 min incubation of free drug or liposomal drugs with: HEPES/sodium chloride buffer at pH 7.4 and 20°C (RT), Triton X-100 in HEPES/ sodium chloride buffer at pH 7.4 and 42°C (TX, pH 7.4) or Triton X-100 in citrate/sodium chloride buffer at pH 4.0 and 42°C (TX, pH 4.0). c) Dox fluorescence of ASDox-LTSLs, and CuDox-LTSLs after 10 min incubation at 42°C at pH 4.0 in the absence of Triton X-100 as a percentage of the fluorescence measured with 0.25% Triton X-100 at pH 4.0. Release by heat was equivalent to release by Triton-X-100. d) CuDox-LTSLs were incubated in citric acid/NaCl buffer, pH 4.0, at 42°C to dissociate the released CuDox to free Dox. Drug release is presented as a percentage of the Dox fluorescence of free Dox at the same concentration under the same conditions. Statistical analyses were performed using one-way ANOVA followed by a Tukey Post Hoc test in (a) and (b) and using a Student’s t-test in (d). **p < 0.01, ***p < 0.001.