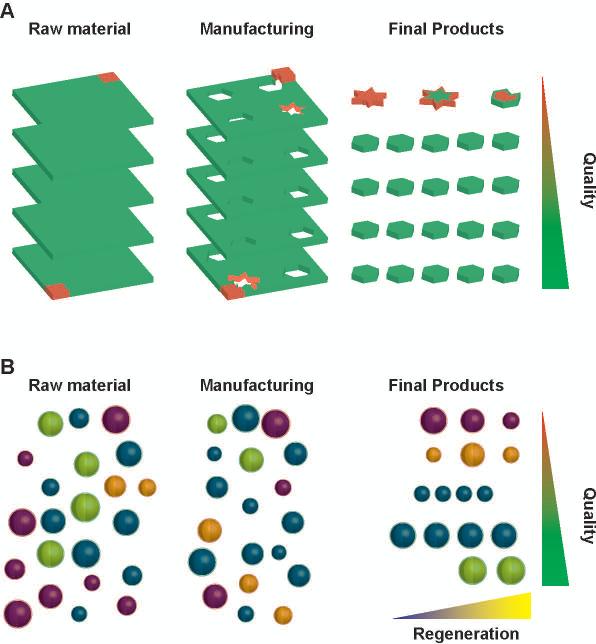
Figure 1. Quality control in sourcing and manufacturing.
Schematics of the quality control practices in traditional (A) and stem cell (B) manufacturing. The amounts of good (green) and defective (red) products in each phase is pre-determined through complete qualification of materials (fill color) and manufacturing (edge color) steps. Unfortunately, patient-specific pools of regenerative cells are sorted based on incomplete characterization, such as the expression of surface markers (outer shell), as key parameters underlying regenerative (yellow core) and non-regenerative (blue core) phenotypes are difficult to characterize. Further how manufacturing and the interaction with the host affect the regenerative cell phenotype is also unclear. As a result, manufacturing of cGMP-grade non-regenerative cell products for regenerative medicine application may occur.