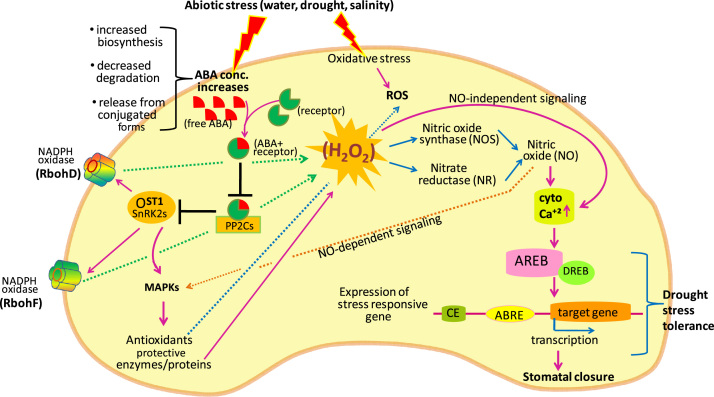
Fig. 2.
During stress condition, ABA accumulates in the guard cells via different ways. It enters into guard cells via ABA transporters, synthesized in response to signals like increased ROS, or accumulates as a result of decreased degradation or release of ABA from conjugated sources. The accumulated ABA interacts with the PYR/PYL/RCAR receptor, and inhibits the PP2Cs, which will result into activation of OST1 and phosphorylation and activation of NADPH oxidase (Rboh). NADPH oxidase facilitates H2O2 generation via signaling pathway·H2O2 induces NO generation by nitric oxide synthase (NOS)-like enzyme(s) and nitrate reductase (NR) that result in the opening of ROS-regulated Ca2+ channels. NO enhances antioxidant gene and enzyme activity via MAPK signaling pathways. H2O2 directly induces ROS-regulated Ca2+ channels (NO-independent signaling) thereby increasing Ca2+(Cyt). Elevated Ca2+(Cyt) induces the expression of abscisic acid–responsive element binding (AREB) protein that binds to the ABA–responsive element (ABRE) motif in the promoter region of ABA-inducible genes. The expression of ABA-responsive genes requires a combination of an ABRE and a coupling element (CE) for a functional promoter. AREB also interacts physically with dehydration responsive element binding (DREB) proteins for the expression of stress responsive genes, leading to stomatal closure under drought conditions.