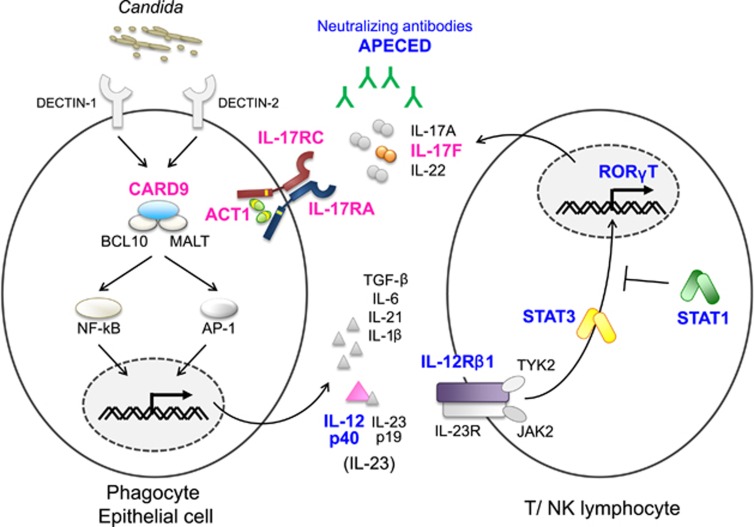
Figure 1.
Inborn errors of IL-17 immunity. Phagocytes recognize C. albicans via pattern recognition receptors and produce proinflammatory cytokines, such as IL-6 and IL-23. These proinflammatory cytokines activate T cells via STAT3 and upregulate RORγT expression, leading to production of IL-17A, IL-17F and IL-22. Impairment in IL-23-induced STAT3-mediated signaling in AD HIES and AR IL-12Rβ1 and IL-12p40 deficiencies cause Syndromic CMC. Neutralizing autoantibodies against IL-17A, IL-17F and IL-22 in patients with APECED impair IL-17 signaling, underlying Syndromic CMC. Patients with AR RORγT deficiency show developmental defects of Th17 cells, resulting in Syndromic CMC. They also develop MSMD, probably caused by impairment of IFN-γ production associated with mycobacterial infections. AD STAT1 gain-of-function was originally identified as a genetic etiology of CMCD. However, it can be categorized as Syndromic CMC based on its broad clinical manifestations. The majority of patients with GOF-STAT1 display a decreased frequency of IL-17-producing cells. Defects in four genes (encoding IL-17F, IL-17RA, IL-17RC and ACT1) that are directly involved in IL-17 signaling have been identified in patients with CMCD. Blue: Syndromic CMC-related molecules and neutralizing antibodies (APECED). Magenta: CMCD-related molecules.