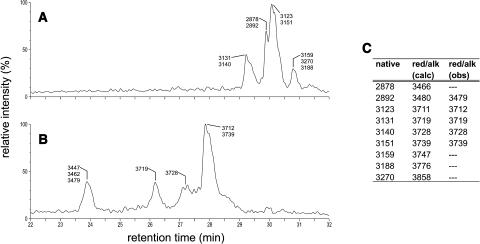
Figure 2.
Effects of Disulfide Bond Modification on the LC-MS Profile of Cyclotides.
LC-MS profile of a native cyclotide fraction (A). After reduction and alkylation of the disulfide bonds (B), the modified cyclotides elute significantly earlier and show masses that correspond to six alkylated half-cystines, providing proof that three disulfide bonds have been broken. (C) provides a tabular listing of the masses observed in (A) (column native) together with the calculated [red/alk (calc)] and observed [red/alk (obs)] masses of the reduced and alkylated peptides. Note that reduction and alkylation of such a complex reaction mixture can lead to incomplete reaction of some species; therefore, masses corresponding to some native cyclotides found in (A) are missing in (B) and (C).