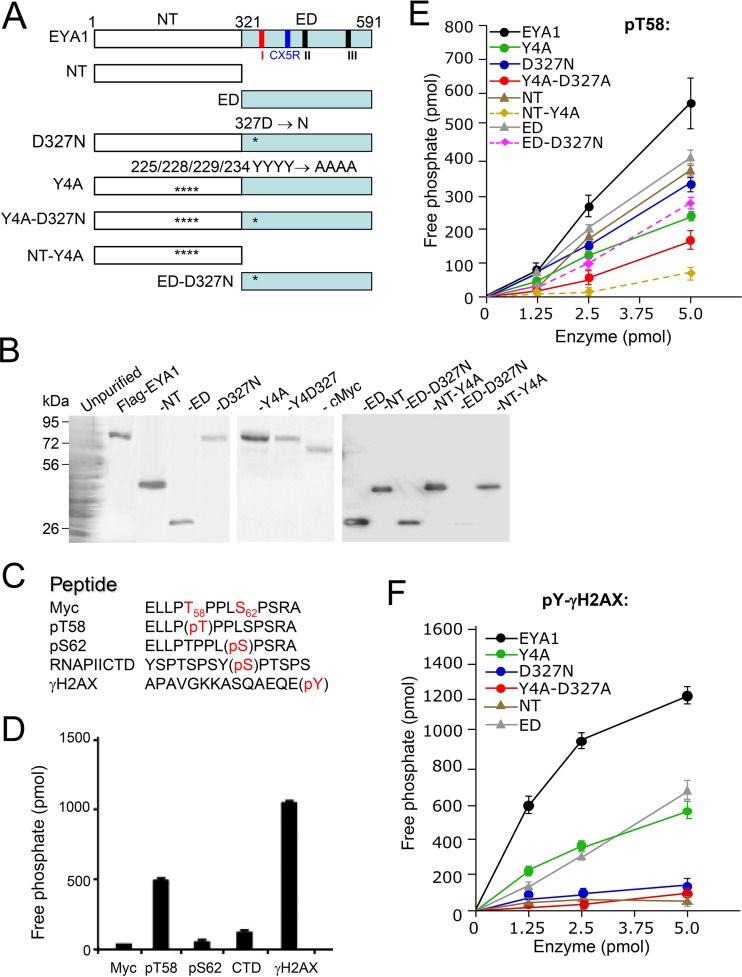
FIG 1.
The conserved C-terminal haloacid dehalogenase EYA domain has dual tyrosine and threonine phosphatase activities. (A) Diagrams of EYA1 and its mutants used for this study. Three short signature motifs for the phosphatase subgroup of the haloacid dehalogenase superfamily (1–3, 7) are marked: motif I (aa 327 to 331), DXDXT/S; motif II (aa 497 to 500), hhh(T/S), where “h” is a hydrophobic residue; and motif III (aa 533 to 559), K(Xn)-hhhhGDXXX(D/E), which is less conserved. Among the three short motifs, the first motif, DXDXT/S (red), is also highly conserved in aspartate-based Ser/Thr phosphatase family members (7). Another signature catalytic motif, CX5R (CXXXXXR; aa 532 to 559), conserved in DUSPs, is marked in blue (27). Asterisks indicate the location of the D327N mutation in the ED. (B) Coomassie staining of an SDS-PAGE gel showing the purified FLAG-EYA1 wild type and its mutant proteins and cMyc proteins from HEK293 cells using anti-FLAG M2 beads. (C) Nonphosphorylated and phospho-Myc peptides, as well as control peptides phospho-S2-RNA PII CTD and phospho-Y-γH2AX, used for enzymatic assays. Phosphorylation modification of amino acid residues (pS/T/Y; red) is shown. (D) Dephosphorylation of different peptides by EYA1. (E and F) Dephosphorylation of pT58-Myc (E) or pY-H2AX (F) peptide by EYA1 and its mutants. All experiments were performed in triplicate, and the error bars represent standard deviations (SD).