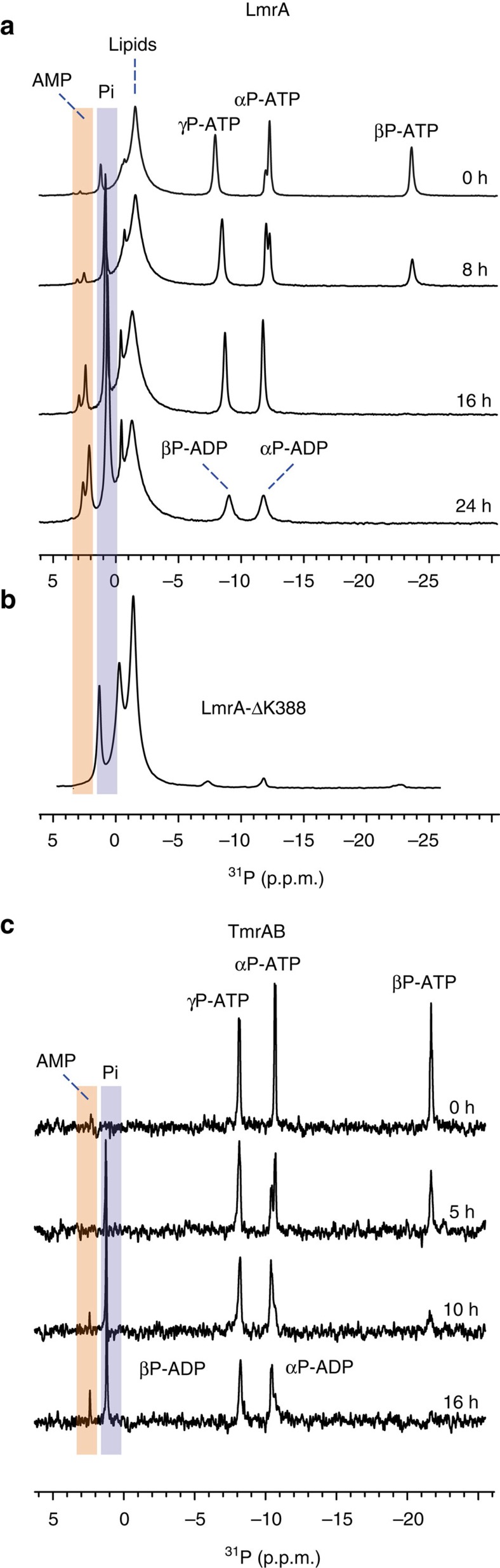
Figure 7. Time-resolved 31P-NMR spectra of LmrA and TmrAB in the presence of ATP reveal AMP formation as observed for MsbA.
(a) 31P-MAS NMR spectra of LmrA proteoliposomes (DMPC/DMPA) show that ATP and ADP are consumed over time to yield Pi and AMP, the latter being the kinase activity marker. (b) AMP formation is not observed in the Walker A Lysine deletion mutant LmrA-ΔK388. This spectrum, recorded at the end of the reaction, was reported by our lab before and is shown here only for comparison57. The slightly different Pi chemical shifts in a,b are because of the choice of different buffers. (c) 31P-solution state NMR spectra of TmrAB in detergent micelles (DDM). At 341 K, formation of Pi and AMP is observed over time. The apparently reduced formation of AMP could be contributed to the heterodimeric nature of TmrAB and its lack of one canonical site for ATP hydrolysis.