Summary
Objectives
The objectives of the study are to characterize the frequency and size of small weight gains during behavioural weight loss treatment and to evaluate the relationship between small weight gains and weight loss outcomes.
Methods
Participants (n = 281) in a year‐long behavioural weight loss programme were weighed at treatment sessions, and between‐session weight gains were classified into several categories based on size. The occurrence of different gain magnitudes and their relation to weight loss were examined during both the active weight loss (months 1–6) and weight loss maintenance (months 7–12) phases of treatment.
Results
Weight gains were common during both phases of treatment, with smaller gains occurring more frequently than larger gains. Greater frequency of all gain magnitudes was associated with lesser weight loss during both phases. Additionally, participants who had just one or two weight gains of the smallest size examined (1.0–1.9 lb) lost less weight than those who had no gains.
Conclusions
Small gains appear to reflect true weight gain due to poor adherence to behavioural recommendations and are associated with worse weight loss outcomes, even when limited in number. Future research should examine how best to prevent small weight gains from occurring and how clinicians and participants should respond when a weight gain does occur to promote weight control success.
Keywords: Behaviour therapy, clinical practice, weight change, weight gain
Introduction
Although behavioural weight loss (BWL) programmes are effective in producing mean weight losses that are clinically significant (i.e. 5–10% of initial body weight) 1, a considerable subset of patients fail to meet weight loss targets. One analysis of weight loss outcomes across several large BWL trials revealed that 38% of participants achieved weight losses of less than 5% 1 year after starting treatment and 26% of participants had weights at or above their starting weight 2. Poor adherence to behavioural recommendations (e.g. calorie and exercise prescriptions) is believed to be a primary cause of suboptimal outcomes 3. Empirical research on how frequently individuals in BWL treatment experience small weight gains and how strongly these gains relate to weight loss outcomes is lacking. Additional research on this topic is needed to determine whether small weight gains during treatment are valid markers of difficulties in adherence to behavioural recommendations, as well as to inform clinical responses to small weight gains.
At present, many standard BWL programmes, such as those based off of the Diabetes Prevention Programme 4 and LEARN 5, do not provide guidance on how to view or respond to small weight gains that occur during active weight loss (vs. weight loss maintenance) attempts. Given the absence of clinical recommendations or empirical data on small weight gain occurrence during active weight loss efforts, clinicians may be uncertain about how concerned to be when a small weight gain occurs during this phase of treatment. For example, clinicians may be unsure if an increase in measured weight reflects true weight gain that is due to a failure to adequately restrict caloric intake or to low activity levels, or whether it may be due to another factor, such as fluid retention or measurement error. Given this uncertainty, clinicians may be apt to wait for a pattern of weight gain to emerge before taking action. If most small gains are due to poor adherence to weight control behaviours, this delayed response may hinder weight loss outcomes. On the other hand, small weight gains may be a normative part of the weight loss process and, when limited in number, may not meaningfully impede outcomes. In fact, it is possible that small weight gains provide opportunities for skill building (e.g. learning how to more effectively plan for high‐risk situations) that promote greater success with weight loss.
There is greater clinical agreement that small weight gains are normative during weight loss maintenance efforts. In fact, many treatment programmes teach participants to anticipate small weight gains during this phase of treatment, frame reversing small weight gains as an important skill for successful long‐term weight control and teach participants specific strategies to use to reverse weight gains during this phase 6, 7, 8. Research on self‐weighing also supports regular and frequent (e.g. weekly and daily) self‐weighing across weight management efforts 9, 10, as obtaining frequent weight measurements via self‐weighing may allow participants to quickly detect small gains and make necessary behaviour changes to reverse small gains 11. These self‐weighing recommendations thus also recognize that there will be some variability in weight measurement and emphasize the importance of immediately identifying small gains in order to prevent larger ones. Despite the presumed importance of responding quickly to reverse small weight gains, little research has investigated the rate at which small weight gains occur or the relationship between small weight gain frequency and weight change during the weight loss maintenance phase of BWL programmes.
The present study aimed to address gaps in the literature about small weight gain occurrence during BWL treatment. First, we characterized small weight gain occurrence during both the active weight loss phase and weight loss maintenance phase of a BWL programme. Second, we examined the relationship between the frequency of small weight gains of several magnitudes and weight loss outcomes during the concurrent treatment phase (loss or maintenance). We hypothesized that more frequent gains of all sizes would relate to lesser weight loss during both phases of treatment. We also examined whether attendance moderated this relationship, with the hypothesis that the inverse relationship between weight gain frequency and weight loss would be stronger among individuals with greater attendance owing to an enhanced ability to capture the true relationship between gains and weight loss given the greater number of weight measurements. Finally, we compared weight losses among individuals who experienced no vs. a very small number of weight gains in an effort to examine the impact of small weight gain occurrence at the lowest threshold that they would be expected to impact outcomes.
Methods
Participants
The current study was a secondary data analysis conducted as part of a larger BWL trial (R01 DK092374). Adults who were overweight or obese were recruited through radio advertisements, flyers and healthcare providers to participate in a BWL programme. Participants were required to have a body mass index between 27 and 45 kg m−2, to be between 18 and 70 years of age and to be able to engage in physical activity. Exclusion criteria included pregnancy, recent weight loss ≥5%, current or history of an eating disorder, history of bariatric surgery and use of certain medications (e.g. insulin). The institutional review board at the supporting institution approved this study. A total of 281 individuals participated in the present study. Two participants who participated in the parent study were excluded from present analyses owing to attending only one session, which precluded any opportunities for experiencing small weight gains. The sample had an average age of 53.3 years (SD = 9.7) and an average body mass index of 35.0 kg m−2 (SD = 4.8 kg m−2) at treatment start.
Procedures
The first 6 months of the treatment (Phase 1) focused on weight loss and consisted of 16 weekly and 4 biweekly sessions. The second 6 months of treatment (Phase 2) consisted of six monthly sessions and focused on weight loss maintenance skills. Participants were randomized to one of three treatment conditions, all of which were based on standard lifestyle modification programmes (Diabetes Prevention Programme/Look AHEAD) and focused on the three key components of BWL (i.e. calorie restriction, physical activity and self‐monitoring). Treatment conditions did not differ on attendance, frequency of weight gains of any size or percent weight loss during either Phase 1 or 2 (ps > 0.12, s < 0.02). Attrition at the beginning of Phase 2 also did not differ significantly between conditions (χ 2 (2, N = 281) = 0.64, p = 0.73). Consequently, treatment conditions were collapsed for all analyses; for additional details of the treatment, refer to Kerrigan et al. 12.
Measures
Anthropometric measurements included body weight, which was measured using a Seca® scale (Seca GmbH & Co, Hamburg, Germany) accurate to 0.1 kg (measured in light street clothes), and height, measured using a stadiometer. Age was self‐reported prior to treatment.
Weight was measured by a clinician in a private room using a Seca® scale accurate to 0.1 kg (in light street clothes). Weights were measured at either a regularly scheduled treatment session (which was held at a constant time from session to session), or, in the case of a participant absence from the regularly scheduled session, at an individual make‐up session with a clinician (which could occur at variable times). Weight change between each attended or make‐up session was calculated, and weight gains were categorized as follows: ≥1.0, 1.0–1.9, 2.0–2.9, 3.0–3.9 and ≥4.0 lb. Two sets of weight change variables were calculated and used in analyses: (i) weight change variables using only weights obtained at regularly scheduled treatment sessions (which were believed to have less potential for measurement error because of consistent time of weight measurement) and (ii) weight change variables using weights obtained from both regularly scheduled treatment sessions and make‐up sessions. Weight gains of <1.0 lb were not examined owing to the increased possibility that gains of this size could be due to measurement error (e.g. due to differences in clothing). We chose to examine gains of several magnitudes ≥1.0 lb given the lack of prior research in this area and the possibility that weight gains of different sizes would relate differentially to weight loss.
If participants had missed a previous session(s), the most recent prior weight was used to calculate weight change, with make‐up weights being considered missing when calculating weight change variables using only weights from regularly scheduled treatment sessions. Weight gains occurring across missed sessions and between biweekly and monthly scheduled sessions were not adjusted to account for differential time spans between weight measurements. Rather, all weight gain magnitudes were calculated based on differences between subsequently measured weights. We chose not to adjust gain magnitudes because of uncertainty about weight trajectories during weeks where participants' weights were not measured. Consequently, weight gains represent changes in weight from session to session and are not necessarily equivalent in meaning between Phases 1 and 2.
Statistical approach
Data were analysed in spss 22.0. Participants were required to have attended two or more treatment sessions during Phase 2 to be included in Phase 2 analyses (n = 220). Last observation carried forward methods were used to account for missing weights at the end of Phase 1 (i.e. session 21) and at the end of treatment (i.e. session 26). We selected this method of handing missing data over other potential methods (e.g. multiple imputation) because of the sensitivity of the present research questions to gains that may otherwise falsely result from imputation.
We used multiple linear regression to examine the relations between small weight gain frequency (for each weight gain magnitude) and percent weight loss during both Phases 1 and 2. Several weight gain frequency variables had small ranges (i.e. less than six gains), were extremely skewed and were not able to be transformed to achieve normality. For example, 228 participants experienced zero 3.0–3.9 lb gains during Phase 1, 46 participants experienced one 3.0–3.9 lb gain and only 7 participants experienced two 3.0–3.9 lb gains. Because we would be unable to capture a linear relationship between these variables and an outcome variable (e.g. percent weight loss), we treated these variables as categorical rather than continuous predictors in analyses. When creating categorical gain frequency variables, we attempted to create as many groups as possible while also striving for as much equivalence in cell sizes as possible. Categorical predictors with more than two weight gain frequency groups we dummy coded for analyses, with the zero gain group serving as the reference category. We included attendance in all models to account for varying numbers of measured weights (and thus opportunities to demonstrate a gain). Attendance during Phase 1 was ln transformed to meet model assumptions. We tested for interactions between attendance and weight gain frequency in predicting percent weight loss for both phases; variables were centred prior to computing interaction terms. We used weighted least squares regression, rather than ordinary least squares regression, for models with non‐constant variance in errors 13.
We used ancovas controlling for attendance to compare weight loss among participants who had no or a very limited number (i.e. one or two) of 1.0–1.9 lb gains and no gains of any other size during both Phases 1 and 2 to try to determine the lowest threshold at which small gains may impact weight loss. We used a Bonferroni correction for post hoc analyses. For Phase 1, only participants who attended a certain number of sessions (i.e. >10) were included in this specific subset of analyses owing to the importance of participants having limited gains despite sufficient opportunity to demonstrate gains (vs. having limited gains largely because of attending only a few sessions) for this particular research question. We selected a cut‐off of 10 attended sessions for inclusion because attendance among the subgroup of participants having zero, one or two 1.0–1.9 lb gains and no others during Phase 1 demonstrated a bimodal distribution with a clear separation occurring at a value of 10. This suggested that using 10 attended sessions as a cut‐off for inclusion in analyses would likely distinguish between participants who experienced limited gains because of poor attendance and those who experienced limited gains while being engaged in treatment. Using a cut‐off of 10 attended sessions also ensured that all participants included in this specific subset of analyses attended at least half of the sessions during Phase 1. A total of 94 participants were included in this subset of analyses. Participants who attended at least three sessions during Phase 2 (to ensure opportunity for demonstrating two gains) and who had zero, one or two 1.0–1.9 lb gains and no other gains of any other size during Phase 2 were included in Phase 2 analyses (n = 107). In addition to comparing measured percent weight loss between these groups, we also conducted an analysis in which we artificially adjusted percent weight loss values for participants who experienced one or two 1.0–1.9 lb gains by subtracting out the number of pounds gained during the one or two gains. This was carried out to determine if differences in weight loss were observed above and beyond the effect of the gains(s) themselves.
Results
A similar pattern of results was observed when running analyses using weights obtained only at regularly scheduled treatment sessions as when using weights obtained from both regularly scheduled and make‐up sessions. Because of less potential for measurement error, we report only on the analyses using weights from regularly scheduled treatment sessions. During Phase 1, mean weight loss was 9.41% (SD = 5.99%, range = 1.81% gain to 27.80% loss) and mean attendance was 15.85 sessions (SD = 4.20, range = 2 to 20). During Phase 2, mean additional weight loss from the start of Phase 2 was 1.25% (SD = 3.78%, range = 8.91% gain to 10.41% loss) and mean attendance was 4.66 sessions (SD = 1.11, range = 2 to 6). Of note, a substantial portion of participants were weighed at either the last or the last or second to last session of both Phase 1 (67.97% and 82.21%, respectively) and Phase 2 (80.45% and 91.36%, respectively), indicating that most participants remained engaged in treatment throughout both phases and provided weight measurements at or near the end of the phase. Attendance was positively related to percent weight loss during Phase 1 (r = 0.53, p < 0.001) and Phase 2 (r = 0.24, p < 0.001). Pearson correlations (for continuous gain variables) and anovas (for categorical gain variables) revealed that attendance differed between gain frequency groups for ≥1.0 and 1.0–1.9 lb gains during Phase 2 (Fs > 3.26, ps < 0.05); no other significant relations/differences for attendance and gain frequency were observed (ps > 0.05).
Characterizing small weight gain occurrence
Table 1 presents descriptive information about the frequency of weight gains of each magnitude for both Phases 1 and 2.
Table 1.
Descriptive information for weight gains of each size by treatment phase
| Size of weight gain (lb) | Mean (SD) number of gains per participant | Range | Percent of participants with at least one gain of this size (%) | Comparison groups used for categorical lapse frequency variables | |
|---|---|---|---|---|---|
| Phase 1 (n = 281) | |||||
| ≥1.0 | 2.08 (1.67) | 0 to 8 | 80.10 | — | |
| 1.0–1.9 | 1.13 (1.17) | 0 to 6 | 64.77 | — | |
| 2.0–2.9 | 0.56 (0.85) | 0 to 5 | 38.79 | 0, 1, ≥2 | |
| 3.0–3.9 | 0.21 (0.47) | 0 to 2 | 18.86 | 0, ≥1 | |
| ≥4.0 | 0.17 (0.42) | 0 to 2 | 15.66 | 0, ≥1 | |
| Phase 2 (n = 220) | |||||
| ≥1.0 | 1.10 (1.02) | 0 to 5 | 65.00 | 0, 1, 2, ≥3 | |
| 1.0–1.9 | 0.42 (0.62) | 0 to 3 | 35.00 | 0, 1, ≥2 | |
| 2.0–2.9 | 0.20 (0.43) | 0 to 2 | 18.64 | 0, ≥1 | |
| 3.0–3.9 | 0.22 (0.47) | 0 to 2 | 19.55 | 0, ≥1 | |
| ≥4.0 | 0.26 (0.55) | 0 to 3 | 21.36 | 0, ≥1 | |
Relations between small weight gain occurrence and weight loss
Table 2 displays results from all regression models examining the relationship between frequency of small weight gains of each magnitude and percent weight loss during Phase 1. Table 1 presents the comparison groups used for all categorical gain frequency variables. As shown in Table 2, there was a significant main effect of weight gain frequency, as well as a significant interaction between gain frequency and attendance, for all models pertaining to Phase 1. For simplicity of interpretation, we present only values from final models. For the models where gain frequency was examined continuously (i.e. for models looking at frequency of ≥1.0 and 1.0–1.9 lb gains), there was a significant interaction between gain frequency and attendance in predicting percent weight loss, such that the strength of the inverse relationship between gain frequency and percent weight loss was attenuated as attendance decreased and increased as attendance increased (Figure 1). A similar pattern was observed for models where gain frequency was examined categorically. For example, percent weight loss increased more among participants who had zero 3.0–3.9 lb gains during Phase 1 as attendance increased than it did among participants who had one or more 3.0–3.9 lb gains (Figure 2).
Table 2.
Frequency of weight gains of all sizes predicts percent weight loss during Phases 1 and 2
| Model | |||||
|---|---|---|---|---|---|
| Size of weight gain (lb) | Predictors | b (SE) | R 2 | F | |
| Phase 1 | |||||
| ≥1.0† | Gain frequency | −0.02 (0.001)*** | 0.55 | 110.73*** | |
| Gain frequency × attendance | −0.01 (0.002)*** | ||||
| 1.0–1.9† | Gain frequency | −0.02 (0.002)*** | 0.46 | 77.34*** | |
| Gain frequency × attendance | −0.02 (0.003)*** | ||||
| 2.0–2.9† | 1 gain group | −0.03 (0.006)*** | 0.41 | 37.67*** | |
| ≥2 gains group | −0.05 (0.007)*** | ||||
| 1 gain group × attendance | −0.02 (0.008)* | ||||
| ≥2 gains group × attendance | −0.02 (0.011)* | ||||
| 3.0–3.9† | ≥1 gain group | −0.04 (0.006)*** | 0.41 | 65.19*** | |
| ≥1 gain group × attendance | −0.02 (0.008)** | ||||
| ≥4.0 lb† | ≥1 gain group | −0.04 (0.008)*** | 0.41 | 64.19*** | |
| ≥1 gain group × attendance | −0.02 (0.010)* | ||||
| Phase 2 | |||||
| ≥1.0 | 1 gain group | −0.03 (0.004)*** | 0.60 | 81.26*** | |
| 2 gains group | −0.05 (0.004)*** | ||||
| ≥3 gains group | −0.10 (0.006)*** | ||||
| 1.0–1.9† | 1 gain group | −0.02 (0.005)*** | 0.16 | 14.16*** | |
| ≥2 gains group | −0.04 (0.008)*** | ||||
| 2.0–2.9† | ≥1 gain group | −0.03 (0.005)*** | 0.20 | 27.45*** | |
| 3.0–3.9† | ≥1 gain group | −0.02 (0.005)*** | 0.13 | 15.73*** | |
| ≥4.0† | ≥1 gain group | −0.05 (0.005)*** | 0.30 | 46.08*** | |
Note. Attendance also was a significant predictor in all models at p < 0.01.
Weighted least squares regression used.
p < 0.05.
p < 0.01.
p < 0.001.
Figure 1.
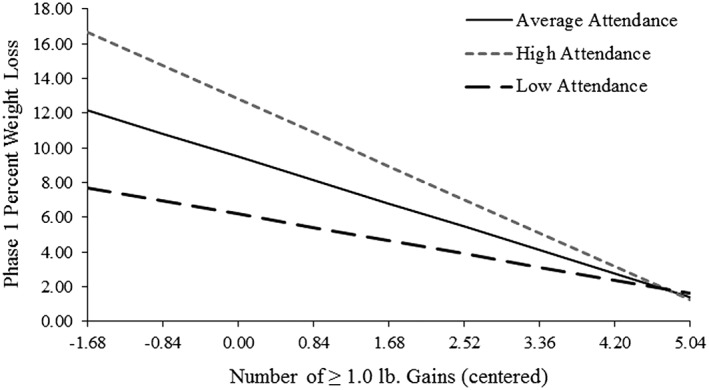
Interaction between ≥1.0 lb gain frequency and attendance in predicting percent weight loss during Phase 1. Note. Low and high attendance reflect ±1 standard deviation, respectively. Attendance was ln transformed and centered. Gain frequency also was centered. Thus, a gain frequency value of 0 depicts mean number (i.e. 2.08) of ≥1.0 lb gains, with ± 0.5 standard deviations depicted along the x‐axis.
Figure 2.
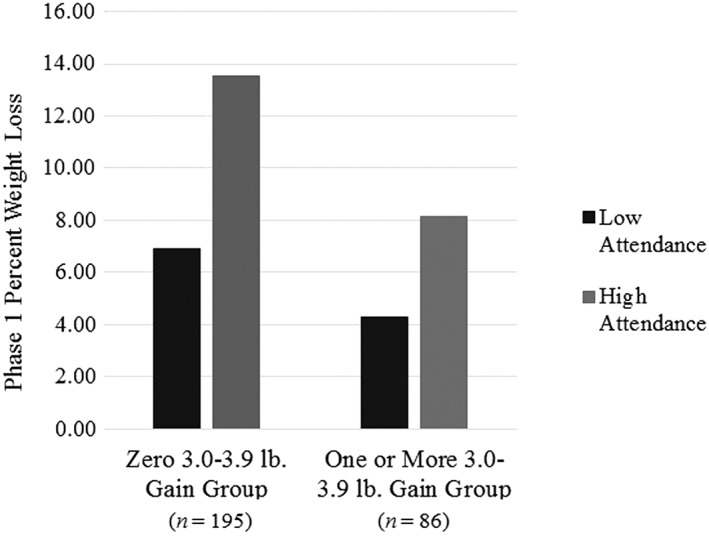
Interaction between 3.0 and 3.9 lb gain frequency group and attendance in predicting weight loss during Phase 1. Note. Low and high attendance reflect ±1 standard deviation, respectively.
Table 2 also displays results from regression models examining the relations between frequency of small weight gains of each magnitude and percent weight loss during Phase 2. There were no significant interactions between attendance and gain frequency during Phase 2. Rather, for all small weight gain magnitudes, more frequent gains were related to lesser percent weight loss when controlling for attendance.
Weight loss among participants experiencing a limited number of small gains
Table 3 displays the results of ancovas comparing both measured percent weight loss and adjusted percent weight loss (i.e. subtracting out the weight gain attributable to the small weight gain(s) themselves) among participants who had zero, one or two 1.0–1.9 lb gains and no gains of any other sizes during both Phases 1 and 2.
Table 3.
Differences in weight loss among participants who had zero, one or two 1.0–1.9 lb gains and no other gains when controlling for attendance
| Percent weight loss variable | F | p |
|
Estimated marginal means | Post hoc comparisons between gain groups | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 gain group (%) | 1 gain group (%) | 2 gains group (%) | |||||||
| Phase 1 | |||||||||
| Observed | 5.91 | 0.004 | 0.12 | 16.10 | 13.30 | 11.90 | 0 > 1* | ||
| 0 > 2** | |||||||||
| 1 = 2 | |||||||||
| Adjusted | 3.00 | 0.06 | 0.06 | 16.10 | 14.00 | 12.10 | — | ||
| Phase 2 | |||||||||
| Observed | 21.85 | <0.001 | 0.30 | 4.60 | 1.90 | −0.10 | 0 > 1*** | ||
| 0 > 2*** | |||||||||
| 1 = 2 | |||||||||
| Adjusted | 10.78 | <0.001 | 0.17 | 4.60 | 2.60 | 1.50 | 0 > 1*** | ||
| 0 > 2*** | |||||||||
| 1 = 2 | |||||||||
Note. Rows labelled ‘Observed’ present results from models comparing actual percent weight loss based on measured weight. Rows labelled ‘Adjusted’ present results from models where the weight loss variable was adjusted to subtract out the weight gain experienced in the one or two 1.0–1.9 lb gain(s) in the specified time period. Bonferroni corrections were used for post hoc comparisons. Only participants who had zero, one or two 1.0–1.9 lb gains and no other gains of any other size in each phase were included in this subset of analyses (Phase 1: n = 93; Phase 2: n = 107). Positive values for estimated marginal means reflect weight loss. Phase 2 values reflect percent weight change from the start of Phase 2.
p < 0.05.
p < 0.01.
p < 0.001.
During Phase 1, differential weight loss was observed among individuals who had no gains, one gain or two gains (n = 94), with participants who had no gains losing significantly more weight (16.10%) than individuals who had one gain (13.30%) or two (11.90%) gains (Figure 3). When percent weight loss was adjusted to remove the weight gain attributable to the gains themselves, differences in weight loss between individuals who had zero gain, one gain or two gains approached significance (p = 0.06).
Figure 3.
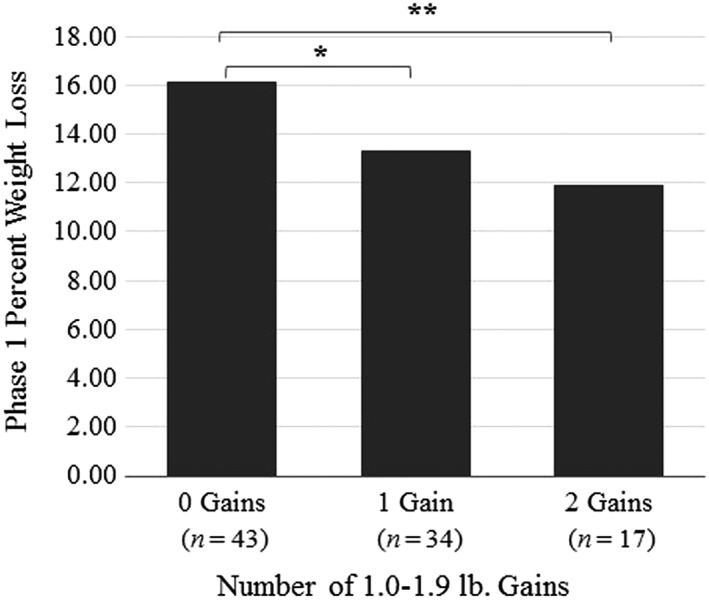
Differences in percent weight loss among participants who had zero, one or two 1.0–1.9 lb gains and no other gains during Phase 1. Note. *p < 0.05, **p < 0.01.
During Phase 2, differential weight loss also was observed among individuals who had no gain, one gain or two gains (n = 107), with participants who had no gains again losing significantly more weight (4.60%) than individuals who had one gain (1.90% loss) or two (0.10% gain) gains (Table 3; Figure 4). After adjustment, individuals who had no gains lost significantly more weight (4.60%) than those who had one gain (2.60%) or two (1.50%) gains.
Figure 4.
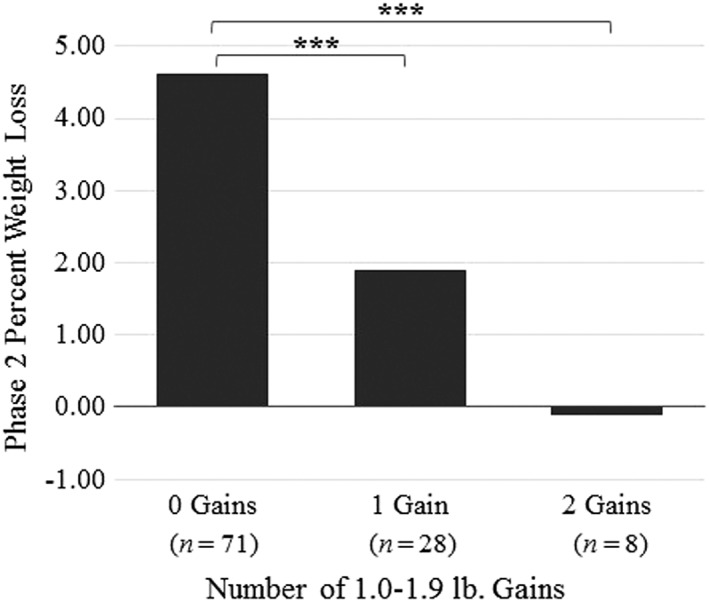
Differences in percent weight loss among participants who had zero, one or two 1.0–1.9 lb gains and no other gains during Phase 2. Note. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The present study characterized small weight gain occurrence and examined the relations between weight gain occurrence and overall weight loss during both the active weight loss phase (Phase 1) and weight loss maintenance phase (Phase 2) of a BWL programme. Results revealed that 80.10% of participants had at least one gain of ≥1.0 lb during Phase 1 and that 65.00% of participants had at least one gain of ≥1.0 lb during Phase 2. Gains that were 1.0–1.9 lb in size were most common during both phases of treatment, with larger gains occurring less frequently, particularly during Phase 1. The finding that 1.0–1.9 lb gains were the most commonly experienced gain magnitude even during Phase 2, when sessions occurred monthly as opposed to weekly or biweekly as in Phase 1, was somewhat surprising, as one might expect larger gains to be more common when gains were calculated over a longer time span between sessions. As indicated by the maximum number of gains experienced for each gain magnitude during Phase 2, some participants experienced gains at all or most sessions during Phase 2. This finding is consistent with past research indicating that many participants regain weight over time 1. Overall, these results suggest that small weight gains, while not ubiquitous, are quite normative during BWL treatment.
Despite being normative, results also revealed that more frequent small weight gains were associated with lesser weight loss. This was true during both phases of treatment. Additionally, during active weight loss, the inverse relationship between the frequency of weight gains (of all sizes) and overall weight loss was stronger among participants who attended more sessions. This finding may indicate that the weight change data that were missing because of unattended sessions obscured the true relationship between weight gain frequency and overall weight change among individuals with poorer attendance and that, had those individuals attended every session, they likely would have exhibited additional weight gains. This possibility is supported by the current and past finding 3, 14 that poorer attendance is associated with lesser overall weight loss. Attendance may not have moderated the relationship between weight gain frequency and overall weight change during Phase 2 owing to the smaller possible difference in attendance across participants during this phase because of fewer overall sessions.
Our results also revealed that there were significant differences in weight loss even among individuals who had no gains, one gain or two gains of the smallest magnitude examined (1.0–1.9 lb) and no other gains – a quite surprising finding. During Phase 1, the observed differences in weight loss between participants who had zero gains, one 1.0–1.9 lb gain or two 1.0–1.9 lb gains appeared to be attributable to the gains themselves. This suggests that individuals who have even a limited number of isolated weight gains during the active weight loss phase of treatment do not catch up to their peers who have no weight gains. During Phase 2, however, differences in weight loss remained between participants who had zero gains vs. one or two 1.0–1.9 lb gains even when accounting for the weight gain(s) themselves. This suggests that participants who had one or two small gains, despite not gaining at other sessions, were less successful in achieving additional weight loss at other sessions compared with participants who had no gains.
There are several implications of the present study. First, the consistent finding that more frequent weight gains are associated with lesser weight loss suggests that the vast majority of observed gains do, in fact, reflect true weight gain that is likely due to poor adherence to behavioural recommendations, as one would not expect to observe differences in overall weight change if gains were due to factors such as fluid retention. Accordingly, session‐to‐session changes in weight should be viewed as valid markers of individuals' adherence (or lack thereof). Second, given that more frequent weight gains of all sizes were associated with lesser weight loss – even when gains were limited in number – clinicians may wish to remain cognizant of the association between small weight gains and weight outcomes when working with participants and making clinical decisions about whether and when to intervene regarding a participant's weight trajectory. Future research is needed to determine the most effective strategies for intervening after a small gain to try to prevent future weight gains. However, discussion of the relationship between small gain occurrence and weight loss outcomes with participants may be warranted to inform participants of the potential threat of small gains to overall success. Although the effect of weighing frequency on weight loss outcomes was not evaluated in the present study, these findings are also consistent with research on the potential value of regular self‐weighing for weight control 9, 10 in that they suggest small weight gains meaningfully relate to longer‐term weight outcomes and that frequent weight measurement may thus be beneficial. While we did not assess daily self‐weighing, the finding that weight outcomes were poorer even among individuals who had only one or two 1.0–1.9 lb gains suggests daily self‐weighing may be most optimal for quickly responding to small changes in weight.
This study has several limitations. One major limitation concerns missing weight data because of unattended sessions. Only weights measured at attended sessions were included in analyses, and last observation carried forward methods were used to account for missing session weights. Although the similar pattern of results observed when using make‐up session weights in analyses suggests that our decision to use only weights from regularly scheduled treatment sessions did not meaningfully affect results, several possible biases remain. First, because last observation carried forward methods were used and weight gain magnitudes were not adjusted when gains occurred across missed sessions, it is possible that some weight gains were improperly categorized with regard to magnitude. For example, if someone gained 1 lb per week but missed a session, he or she would be incorrectly categorized as having experienced a 2 lb gain. We decided not to adjust gain magnitudes across missed sessions to avoid altering gain frequency (i.e. in the aforementioned example, the person would be reported to have two gains rather than one, despite only having one measured weight gain), as well as because of uncertainty about weight trajectory between missed session (i.e. it is possible that the aforementioned individual was weight stable during the first week and gained 2 lb only in the second week). However, this decision may have misrepresented the size of weight gains occurring across missed sessions, particularly with regard to larger gains. Because similar methods were used for gains occurring when regularly scheduled meetings occurred less frequently (i.e. biweekly and monthly), gains should be considered as reflecting session‐to‐session vs. weekly weight change, and future research may wish to examine gains on a standardized, more frequent basis. Additionally, we did not use multiple imputation because we wanted to accurately characterize weight gains based on information that would be available to clinicians providing treatment (i.e. session weights) and therefore increase application of these findings. However, it is possible that findings would differ with use of imputed data.
An additional limitation of this study concerns the relationship between attendance and weight gain occurrence. Although we attempted to address the potential confounding influence of differential attendance on the relationship between weight gains and weight outcomes by examining attendance as a moderator of this relationship, number of gains and attendance are not truly independent variables. Consequently, moderation results should be interpreted cautiously. Additionally, the relationships between attendance, number of gains and weight outcomes are likely quite complex (e.g. because of missing data) and may not be fully captured by the statistical methods used in this study. Additional investigation of these relationships is thus warranted. Finally, weight gains of <1.0 lb were not examined.
Future research should examine whether the timing of gains within each phase relates to weight loss (e.g. are gains that occur later or earlier related to less weight loss?), whether early gains predict later success in treatment (e.g. because of learning opportunity) and how weight variability or weight change trajectories relate to weight loss outcomes. Additionally, while our findings suggest that weight gains are likely due to poor adherence, this study did not explicitly measure adherence to behavioural recommendations (e.g. calorie prescription) or examine how adherence relates to weight gains. Examination of behavioural predictors of weight gains is essential to understand their cause and would be a next step to further these analyses. Future studies investigating the importance of small weight gains in weight control may benefit from using study designs in which participants self‐weigh using ‘smart’ scales. These scales can reliably translate weight data to researchers 15, and studies that have participants weigh themselves at home using these devices would allow for more frequent weight measurements, in addition to reducing potential biases in results because of missing data (e.g. because of missed sessions).
Future research should also examine how best to prevent small weight gains (e.g. by emphasizing consistency and the potential negative impact of even limited small gains) and how best to support participants when gains do occur. For example, investigation of an immediate, concerned response from a clinician at the first small weight gain could illuminate if early intervention mitigates future small gains. Although additional future research is necessary to determine the most effective methods of intervention after a small weight gain, assessing the adequacy of caloric restriction and engaging in problem‐solving to promote renewed or enhanced restriction may be important given the crucial role of caloric restriction in short‐term weight loss 1. Examining characteristics of the group that had no or a limited number of gains could elucidate potential prevention methods of small gains.
In conclusion, the present study suggests that while normative, small weight gains of ≥1.0 lb are associated with lesser weight loss during both the active weight loss‐focused and weight loss maintenance‐focused phases of BWL treatment. Additionally, participants who have even one or two small gains lose less weight than those who have no gains. While additional research on this topic is needed, these findings provide preliminary indication that small weight gains at any point during BWL treatment are cause for concern. Clinicians should thus be aware of the potential long‐term consequences of small gains. With replication and further study of interventions following small gains, BWL programmes may benefit from framing small weight gains as important indicators of potential difficulty with weight control and emphasize the importance of preventing and responding quickly to small weight gains at all points during BWL treatment.
Conflict of Interest Statement
Dr. Butryn and Dr. Forman received funding from the National Institutes of Health during the conduct of the study. Dr. Forman also received research funding from the Obesity Society, Weight Watchers and Shire Pharmaceuticals. All other authors declare no conflicts of interest.
Funding
This research was supported by NIDDK grant R01 DK 092374.
Contributions
L. S. conceived the study (with assistance from M. B.), carried out analyses, prepared figures, and conducted literature searching. M. G. and J. R. assisted with literature searching and table preparation. F. Z. assisted with data analysis and interpretation. M. B. and E. F. conceived and carried out the parent study. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Schumacher, L. M. , Gaspar, M. , Remmert, J. E. , Zhang, F. , Forman, E. M. , and Butryn, M. L. (2016) Small weight gains during obesity treatment: normative or cause for concern?. Obesity Science & Practice, 2: 366–375. doi: 10.1002/osp4.73.
References
- 1. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014; 63 25_PA. [DOI] [PubMed] [Google Scholar]
- 2. Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: using categorical data. Int J Obes 2010; 34: 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Acharya SD, Elci OU, Sereika SM, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence 2009; 3: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diabetes Prevention Program (DPP) Research Group . The Diabetes Prevention Program (DPP) description of lifestyle intervention. Diabetes Care 2002; 25: 2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brownell KD. The LEARN Program for Weight Management. (10th ed.) American Health Publishing Co: Dallas, TX, 2000. [Google Scholar]
- 6. Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003; 24: 610–628. [DOI] [PubMed] [Google Scholar]
- 7. Ross Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long‐term maintenance of weight loss: a systematic review and meta‐analysis. Obes Rev 2012; 13: 509–517. [DOI] [PubMed] [Google Scholar]
- 8. Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21: 323–341. [DOI] [PubMed] [Google Scholar]
- 9. Pacanowski CR, Bertz F, Levitsky DA. Daily self‐weighing to control body weight in adults. SAGE Open 2014; 4: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. VanWormer JJ, French SA, Pereira MA, Welsh EM. The impact of regular self‐weighing on weight management: a systematic literature review. Int J Behav Nutr Phys Act 2008; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self‐monitoring of weight: a key component of successful weight loss maintenance. Obesity 2007; 15: 3091–3096. [DOI] [PubMed] [Google Scholar]
- 12. Kerrigan SG, Schaumberg K, Kase C, Gaspar M, Forman EM, Butryn ML. From last supper to self‐initiated weight loss: pretreatment weight change may be more important than previously thought. Obesity 2016; 24: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garson GD. Weight Least Squares Regression. Statistical Associates Publishers: Asheboro, NC, 2013. [Google Scholar]
- 14. Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight‐loss maintenance trial. Am J Prev Med 2008; 35: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel‐Hodge C, Ward DS. The efficacy of a daily self‐weighing weight loss intervention using smart scales and e‐mail. Obesity 2013; 21: 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]


