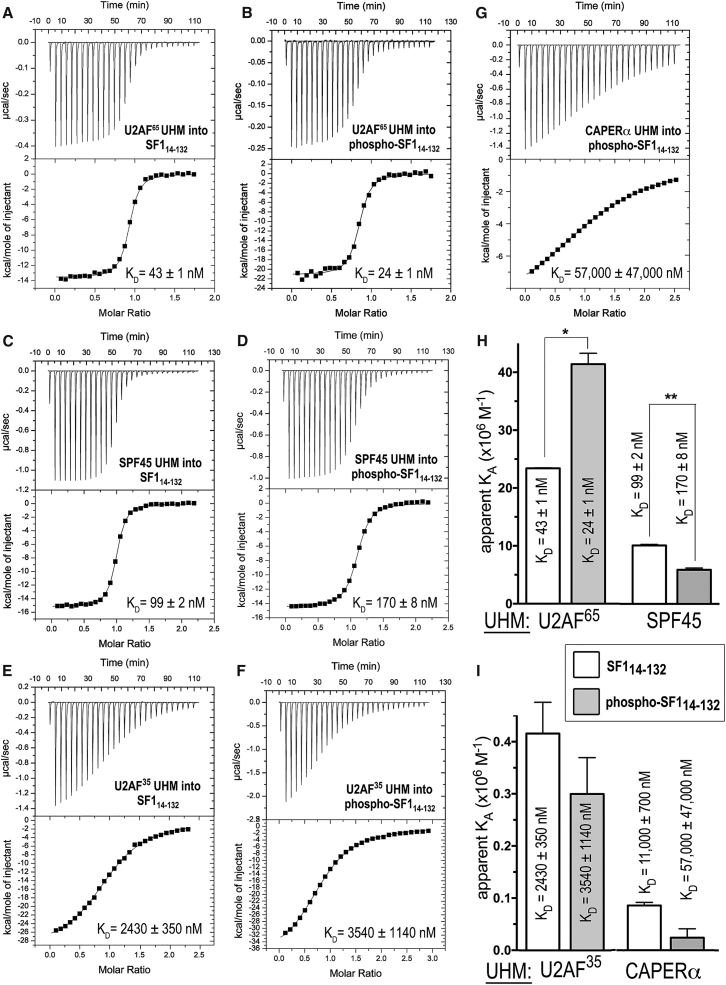
Figure 2.
ITC results for SF114–132 or phospho-SF114–132 titrated with UHM proteins. (A–G) Representative isotherms fit with identical sites models. Titrations were of (A) 90 μM U2AF65 UHM into 10 μM SF114-132, c = 230; (B) 32 μM U2AF65 UHM into 4 μM phospho-SF114–132, c = 170; (C) 200 μM SPF45 UHM into 20 μM SF114–132, c = 200; (D) 200 μM SPF45 UHM into 20 μM phospho-SF114–132, c = 120; (E) 200 μM U2AF35 UHM into 20 μM SF114-132, c = 8; (F) 260 μM U2AF35 UHM into 20 μM phospho-SF114–132, c = 6; and (G) 600 μM CAPERα UHM into 50 μM phospho-SF114–132, c = 1. (H and I) Bar graphs showing the apparent equilibrium affinities (KA) of SF114–132 (white bars) and phospho-SF114–132 (gray bars) for the indicated UHM proteins. The apparent equilibrium dissociation constants (KD = KA−1) and the standard deviations of at least two replicates are given. Values from (G) are estimates due to the low affinity binding, which is at the limit for reliable fits of ITC data.