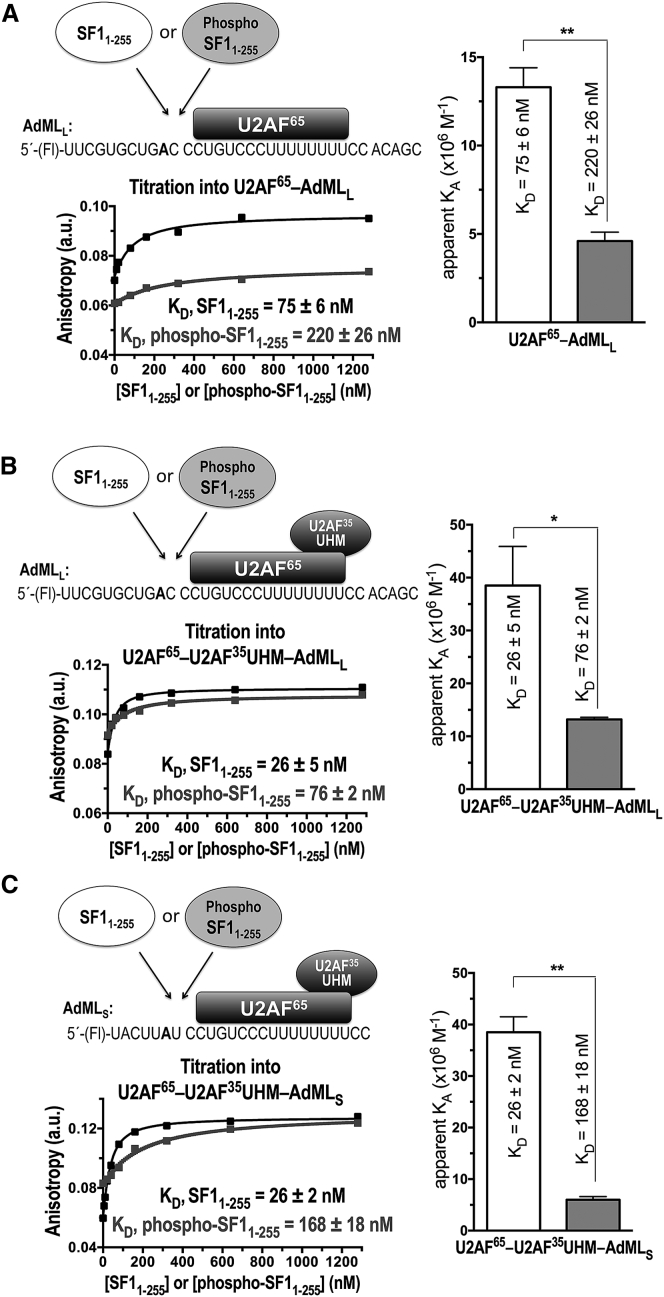
Figure 3.
Fluorescence anisotropy RNA binding assay of SF11–255 (white bars, black binding curve) or phospho-SF11-255 (gray shading and binding curve) proteins titrated into fluorescein (Fl)-labeled AdML RNA oligonucleotides, which were pre-bound to full-length U2AF65 (either with or without the U2AF35 UHM subunit). The SF11–255 or phospho-SF11–255 proteins were titrated into protein-RNA complexes (A) Fl-AdMLL-U2AF65, (B) Fl-AdMLL-U2AF65-U2AF35 UHM, (C) Fl-AdMLS-U2AF65-U2AF35 UHM. Schematic diagrams of the experiments, shown above the plots, provide the RNA sequences (branchpoint in bold). The plots show representative fits of the fluorescence anisotropy changes during titration. Bar graphs of the apparent equilibrium affinity (KA) values are plotted on the right. The apparent equilibrium dissociation constants (KD = KA−1) and the standard deviations of three technical replicates are given.