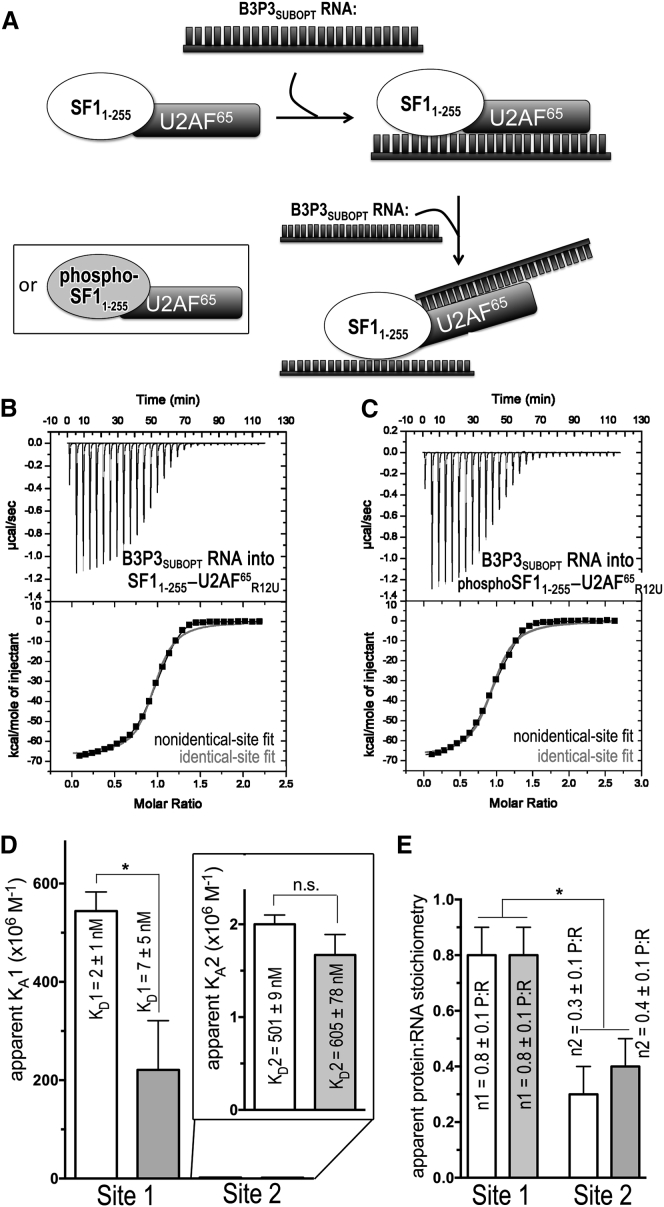
Figure 6.
ITC results for SF11–255-U2AF65R12U (white) or phospho-SF11–255-U2AF65R12U (gray) protein complexes titrated with B3P3SUBOPT RNA. (A) Schematic diagram of the ITC experiment. (B and C) Representative isotherms fit with binding models for either nonidentical sites (black lines) or identical sites (red lines). Titrations were of (B) 35 μM B3P3 RNA into 5 μM SF11–255-U2AF65R12U complex (nonidentical sites, χ2 = 0.20E6 ± 0.16E6; identical sites, χ2 = 2.70E6 ± 0.29E6), c = 5000 for site 1 and c = 10 for site 2; and (C) 35 μM B3P3 RNA into 5 μM phospho-SF11–255-U2AF65R12U complex (nonidentical sites, χ2 = 0.23E6 ± 0.19E6; identical sites, χ2 = 3.13E6 ± 0.95E6), c = 700 for site 1 and c = 8 for site 2. The identical site models were discarded based on the large χ2 increase, poor fit, and nonidentical sites of reverse titrations (Fig. S5). (D) Bar graph of apparent affinities (KA) of the interactions, colored as for (A). The apparent equilibrium dissociation constants (KD = KA-1) and the standard deviations of at least two replicates are given. An expanded view of the lower affinity sites is inset. (E) Bar graph of apparent protein:RNA stoichiometries (n).