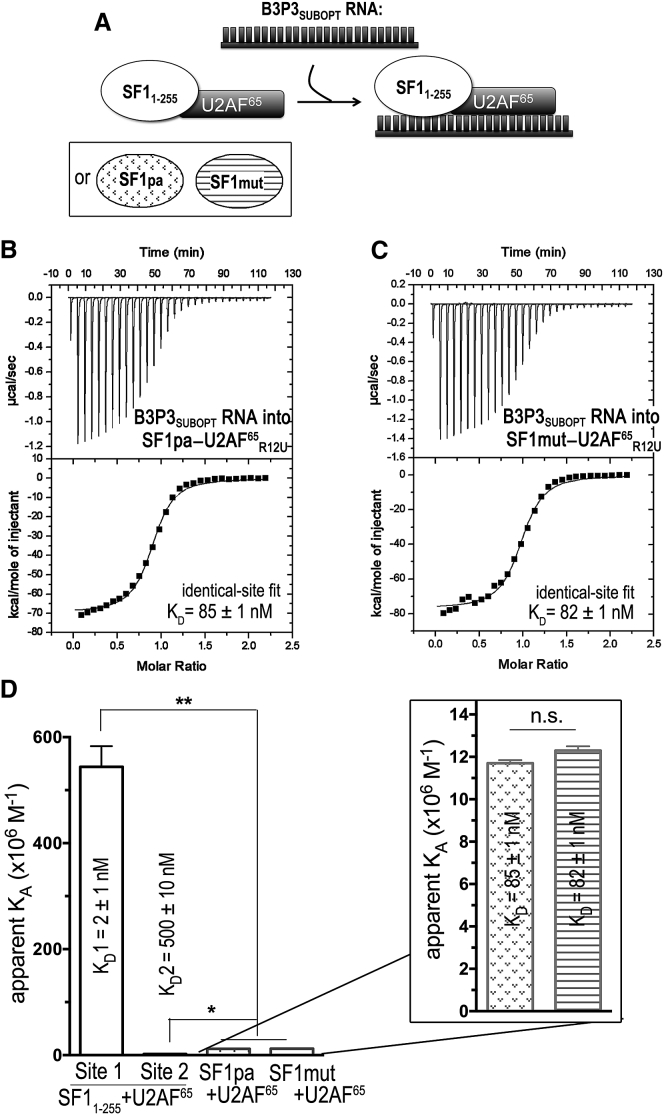
Figure 8.
ITC results for SF1mut-U2AF65R12U (striped) or SF1pa-U2AF65R12U (stippled) complexes titrated with B3P3SUBOPT RNA. (A) Schematic diagram of the ITC experiment. (B and C) Representative isotherms fit with identical site models for (B) 50 μM B3P3 RNA into 5 μM SF1pa-U2AF65R12U complex, c = 59, and (C) 50 μM B3P3 RNA into 5 μM SF1mut-U2AF65R12U complex, c = 61. Nonidentical-site models were discarded based on 1) fitting errors greater than the resulting thermodynamic values; 2) stoichiometry values of nearly zero for one class of nonidentical sites; and 3) thermodynamic values for the remaining class of sites that matched the results of the identical-site fit. (D) Bar graph of apparent affinities (KA) of the interactions, colored as in (A). The apparent equilibrium dissociation constants (KD = KA−1) and standard deviations of at least two replicates are given. For comparison, the nonidentical-site affinities of the parent SF11–255-U2AF65R12U complex titrated with B3P3 RNA (details in Fig. 6) are plotted at left. The inset shows an expanded view of the B3P3 RNA affinities for the SF1mut- or SF1pa-U2AF65R12U complexes. A control titration of B3P3SUBOPT RNA into buffer is shown in Fig. S1 C and a titration of B3P3SUBOPT RNA into SF1mut-U2AF65R12U in phosphate buffer (as opposed to HEPES) is shown in Fig. S6.