Abstract
The timetable of effects on bone repair of the active fraction‐parathyroid hormone, PTH(1‐34), was analytically investigated from the morphometric viewpoint in 3‐month‐old male Sprague‐Dawley rats, whose femurs were drilled at mid‐diaphyseal level (transcortical holes). The animals were divided into groups with/without PTH(1‐34) administration, and sacrificed at different times (10, 28, 45 days after surgery). The observations reported here need to be framed in the context of our previous investigations regarding bone histogenesis (Ferretti et al. Anat Embryol. 2002; 206: 21–29) in which we demonstrated the occurrence of two successive bone‐forming processes during both skeletal organogenesis and bone repair, i.e. static and dynamic osteogenesis: the former (due to stationary osteoblasts, haphazardly grouped in cords) producing preliminary bad quality trabecular bone, the latter (due to typical polarized osteoblasts organized in ordered movable laminae) producing mechanically valid bone tissue. The primary function of static osteogenesis is to provide a rigid scaffold containing osteocytes (i.e. mechano‐sensors) for osteoblast laminae acting in dynamic osteogenesis. In the present work, histomorphometric analysis revealed that, already 10 days after drilling, despite the holes being temporarily filled by the same amount of newly formed trabecular bone by static osteogenesis independently of the treatment, the extent of the surface of movable osteoblast‐laminae (covering the trabecular surface) was statistically higher in animals submitted to PTH(1‐34) administration than in control ones; this datum strongly suggests the effect of PTH(1‐34) alone in anticipating the occurrence of dynamic osteogenesis involved in the production of good quality bone (with more ordered collagen texture) more suitable for loading. This study could be crucial in further translational clinical research in humans for defining the best therapeutic strategies to be applied in recovering severe skeletal lesions, particularly as regards the time of PTH(1‐34) administration.
Keywords: collagen texture, fracture healing, osteoblasts, osteocytes, PTH(1‐34), rat model
Introduction
PTH(1‐34), also known as Teriparatide, is an FDA‐approved anabolic drug for osteoporosis, increasing bone deposition. It has also been demonstrated that, although PTH(1‐34) can also stimulate bone resorption, the net effect of intermittent daily administration is the increase in bone mass (Hock & Gera, 1992; Burr et al. 2001; Oswald et al. 2014; Saidak et al. 2014; Takao‐Kawabata et al. 2015). Under some conditions, PTH(1‐34) can be used to improve fracture healing (Chiang et al. 2013), although this may be dependent on the severity and location of bone injury as well as on an individual's overall bone health.
PTH(1‐34) has been widely used in osteoporotic patients and bone lesion repair in humans, as shown by the great number of papers on clinical studies (Ma et al. 2006; Thomas, 2006; McDonald et al. 2008). However, notwithstanding this, a better knowledge of some aspects of PTH(1‐34) action on bone tissue could be achieved mostly through studying the sequence of events occurring during bone healing in experimental lesions of animal models. Up to now, most investigations on transcortical bone lesions reported in the literature aimed at quantifying bone mass formed during bone repair (Kusuzaki et al. 2000; Chiba et al. 2001; Karp et al. 2004; Pereira et al. 2007; Oana et al. 2008), rather than analyzing the quality of newly secreted bone depending on the type of osteogenesis. As was demonstrated by us for the first time over the last decade in a series of structural and ultrastructural investigations, there exist two different mechanisms of bone formation, occurring in sequence during intramembranous ossification in both physiologic and pathologic conditions (Ferretti et al. 2002, 2006; Palumbo et al. 2003, 2004; Marotti, 2004; Marotti et al. 2010). We named these two processes of bone formation static osteogenesis (SO) and dynamic osteogenesis (DO), respectively, because the former is characterized by pluristratified cords of ‘stationary’ osteoblasts which differentiate by inductive stimuli (Streeten & Brandi, 1990; Villanueva & Nimni, 1990; Kasperk et al. 1997; Inoue et al. 2000) at a fairly constant distance from the network of blood capillaries, without moving during their transformation into osteocytes from the differentiation site; the latter being instead performed by the well‐known typical monostratified laminae of ‘movable’ osteoblasts. The temporal sequence of events is as follows: first, variously polarized stationary osteoblasts are irregularly arranged inside cords and give rise (in the same place where they differentiate) to osteocytes clustered within confluent lacunae, thus allowing the formation of a preliminary trabecular woven bone, not valid from the mechanical viewpoint due to its too‐high random cellularity. Subsequently, dynamic osteogenesis occurs on the surfaces of the woven‐textured SO‐trabecular framework, mainly involved in bone compaction, i.e. filling primary haversian spaces with primary osteons. Generally, in comparison with SO‐trabecular bone, DO‐bone is a lamellar bone that is mechanically more resistant, since it is less microporous and arranged in a more orderly fashion with respect to both the cells and the fibrillar components of its intercellular matrix. Moreover, it is secreted in relation to mechanical stimuli, instead of vascular‐derived inductive factors (as occurs for SO). This aspect acquires particular importance during fracture healing when, as normally occurs in bone histogenesis, static and dynamic osteogenesis are temporally correlated and follow each other (Marotti, 2004).
The present study concerns investigations on the subject of timing and manner of bone healing in rat femur, after drilling transcortical holes, with/without contemporary administration of PTH(1‐34). As the ability of PTH(1‐34) to recover bone mass in patients with osteoporosis has been already demonstrated (Ma et al. 2006; Jilka, 2007; Lindsay et al. 2007) as well as during fracture healing in experimental animal models (Kusuzaki et al. 2000; Skripitz et al. 2000; Chiba et al. 2001; Karp et al. 2004; Pereira et al. 2007; Oana et al. 2008; Jilka et al. 2009), the novelty and the importance of this investigation is the evaluation of the drug effect during the sequential occurrence of the two types of osteogenesis on: (i) the time in which it is most effective; (ii) the type of the microscopic structure of the newly formed bone tissue, lamellar or woven bone, respectively more or less suitable for loading.
The final goal is to identify the phase of osteogenesis (static vs. dynamic) in which PTH(1‐34) can most significantly exert its effect (if any), in order to determine how and when to use the drug with maximum efficacy in improving bone lesion repair strategies.
Materials and methods
Experimental animals and treatment
Twenty‐eight 3‐month Sprague–Dawley male rats (purchased from Charles River Laboratories ‐ Calco, Lecco, Italy) were divided into six groups (after 7 days of acclimation to housing conditions) in which, at mid‐diaphysis of the lateral aspect of left femur, one transcortical hole was drilled using a dental bur, producing a bone defect of 1.5 mm in diameter. The modest dimension of the hole, in line with other authors (Chiba et al. 2001; Pereira et al. 2007) was chosen not only to better manage the drilling (each hole penetrated the cortex to the medullary cavity, but did not penetrate the opposite cortex) but also to reduce healing time with respect to larger bone defects and to allow normal ambulation soon after surgery. All rats were housed individually in single cages and maintained under laboratory controlled conditions (22 ± 1 °C, 55–60% humidity, 12‐h light : 12‐h dark). From the day after the surgery, some animals were also treated with PTH(1‐34), supplied by Eli Lilly and Company (Indianapolis, IN, USA), solubilised in saline (40 μg mL−1) and subcutaneously injected in a volume of 100 μL/100 g body weight per rat, according to the following scheme.
Group 1 (n = 5): drilled animals (as drilled control) sacrificed after 10 days.
Group 2 (n = 5): drilled animals treated with PTH(1‐34) for 10 days and then sacrificed.
Group 3 (n = 5): drilled animals (as drilled control) sacrificed after 28 days.
Group 4 (n = 5): drilled animals treated with PTH (1‐34) for 28 days and then sacrificed.
Group 5 (n = 4): drilled animals (as drilled control) sacrificed after 45 days.
Group 6 (n = 4): drilled animals treated with PTH (1‐34) for 45 days and then sacrificed.
The animals of groups 3, 4, 5 and 6 underwent a subcutaneous injection of 15 mg kg−1 calcein (Fluka, St. Louis, MO, USA) at day 10 after surgery, to highlight the actual newly formed bone.
All experiments were carried out according to the Bioethical Committee of the Italian National Institute of Health and were authorized in accordance with Decrees of the Italian Ministry of Health (protocol numbers 99/2013‐B and 100/2013‐B of 12 April 2013). Animal care, maintenance and surgery were conducted in accordance with Italian law (D.L. no. 116/1992) and European legislation (EEC no. 86/609).
Histology and histomorphometry
Soon after euthanasia, femora were harvested, fixed in 4% buffered paraformaldehyde, dehydrated in a graded series of ethanol and embedded in methylmetacrylate resin (Sigma Aldrich, Milan, Italy). Transversal sections (three from each animal) 5 μm thick of the femora in the region corresponding to the center of the hole were obtained by means of a Reichert‐Jung 1150/Autocut microtome (Walden, Austria) and stained with a Gomori trichrome solution. Some sections were also observed under transmitted polarized light (TPL). Sections for fluorescence microscopy were not stained. The sections were photographed using a NIKON Eclipse 90i microscope (Tokyo, Japan) equipped with a DS‐Fi1 Nikon digital camera and driven by the Nikon ACT‐2U software; histomorphometry was performed by means of the software imagej (NIH, Bethesda, MD, USA). Two blinded researchers performed the histological measurements. The amount of bone deposited within the hole area was evaluated in all experimental groups and expressed as BV/TV in percentage value. In those animals sacrificed 10 days after creation of the bone defect, the extent of the surface of prismatic osteoblast laminae was measured with respect to the bone surface of the healing hole (Ob.S/BS expressed in percentage value) to evaluate the amount of dynamic osteogenesis. In those animals sacrificed 28 days after creation of the bone defect, the values of calcein‐labeled bone area (L B Ar) and the amount of cortical compact bone (Ct B Ar) were measured with respect to the hole area (hole Ar), and both expressed in percentage values. The fluorescent calcein labels were selected with the threshold function of imagej and then measured as area fraction within the hole area.
Micro‐hardness
The micro‐hardness (HV) of the newly formed bone inside the holes was evaluated at 28 and 45 days in all control and treated animals using a Leitz‐Durimet micro‐hardness tester (Wetzlar, Germany) equipped with a Vickers indenter. After obtaining the histological sections, the remaining part of the specimens were polished with Allumina Yellow Solution (Carlo Erba Reagenti, Cornaredo‐Milano, Italy). Six indentations were then performed under a load of 50 g for each specimen in the newly formed bone, and the length of the diagonals of each indentation was measured with a screw micrometric eye‐piece at an enlargement of 400×. The micro‐hardness number (expressed in kg mm−2) was computed according to the formula HV = 1854.4 * L/d2, where (L) is the load in grams and (d) is the mean length of the diagonals of the indentation (measured in microns).
Scanning electron microscopy (SEM)
To visualize the collagen texture of the newly formed bone, some sections (previously used for micro‐hardness evaluation) were observed with the ESEM Quanta‐200 Scanning Electron Microscope under low vacuum condition and in backscattered mode.
Serum biochemical analysis
After death, blood samples were collected by cardiac puncture and serum was immediately separated by centrifugation (4 °C) at 1500 g for 15 min, and then aliquoted into small volumes and stored at −20 °C for successive analyses. The levels of total calcium (Ca) and inorganic phosphorus (P) in serum were determined using the high performance Beckman Coulter analyzer AU680 Chemistry System. The immune‐metric assays for the determination of levels of osteoprotegerin (OPG), specific bone alkaline phosphatase (BALP), CTX (Beta CrossLaps) and Bioactive‐intact‐PTH (1‐84) in rat serum were provided by Pantec s.r.l. (Turin, Italy); all kits are intended for research use only. Rat‐OPG and Rat‐BALP are two ELISA kits produced by SunRed Hotecnology Company (Shanghai, China); RatLaps is an ELISA kit produced by Immunodiagnostic Systems Ltd (Boldon, UK); and Rat Bioactive‐intact‐PTH is an ELISA kit produced by Immunotopics Inc. (San Clemente CA). The small amounts of reagents supplied in the kits precluded the performing of automated procedures on the laboratory analytical platform. To minimize the variables influencing the test, all the principles of good laboratory practice were followed: immediate storage of samples after serum separation at −20 °C; manual execution of tests in close agreement with the manufacturer's instructions; performing of all tests over two consecutive days to avoid repeated freeze/thaw sample cycles.
Statistical analysis
Student's t‐test, using the Software stata 11.0 (StataCorp, TX, USA), was performed to compare means of treated and control groups, according to the time of sacrifice. P‐values of < 0.05 were considered significant.
Results
In the proposed rat model the observations performed during hole repair, going from the periosteal side towards the endosteal one, demonstrated the same sequential phases that occur during fracture healing: after the hematoma and inflammatory stages, (i) the gap is filled with highly vascularized connective tissue; (ii) subsequently, cords of stationary osteoblasts differentiate in between the blood capillaries, giving rise to a trabecular bony framework laid down by SO; (iii) later, typical laminae of movable osteoblasts are differentiated on the surfaces of the SO‐trabeculae and thicken them by DO (Fig. 1).
Figure 1.
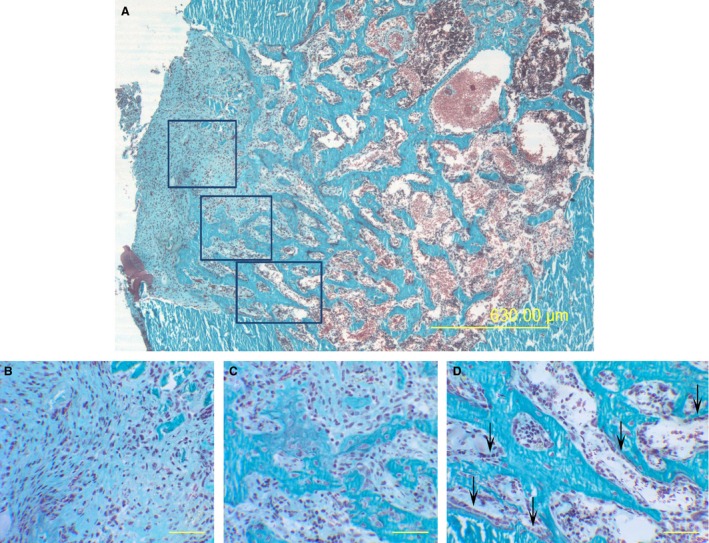
Representative LM micrographs passing through the hole center, from a control rat 10 days after CBD: showing (A) from periosteal side (left) towards the endosteal one (right): highly vascularized connective tissue (enlarged in B), trabecular bony framework laid down by cords of stationary osteoblasts (enlarged in C), typical laminae of movable osteoblasts (arrows) differentiating on the surfaces of such SO‐trabeculae (enlarged in D). (B,C,D) Enlargements of the squared areas in (A). Scale bars: (A) 630 μm, (B,C,D) 50 μm.
Ten days after creation of bone defect
After 10 days from creation of bone defect (CBD), in both control and treated animals the bone gap is filled by trabecular bone, mostly laid down by means of static osteogenesis (Fig. 2A,B). The total amount of newly formed trabecular bone (expressed as BV/TV%) recorded in the areas of healing hole shows no variations in the two animal groups (Table 1). Given the same amount of newly formed bone, different histological features are detectable in control and treated groups. In control animals, cords of pluristratified stationary osteoblasts are abundant and well visible within the fibrous tissue separating the forming bony trabeculae (circles in Fig. 2A). In treated animals, on the other hand, close to the surfaces of trabecular bone deposited by static osteogenesis, well developed laminae of movable osteoblasts (a typical feature of dynamic osteogenesis) were clearly observed (arrows in Fig. 2B). Concerning the amount of dynamic osteogenesis, the extent of the surface of prismatic osteoblast laminae with respect to the bone surface of healing hole (Ob.S/BS%) was statistically higher (P < 0.05) in treated than in control animals (Fig. 2C,D).
Figure 2.
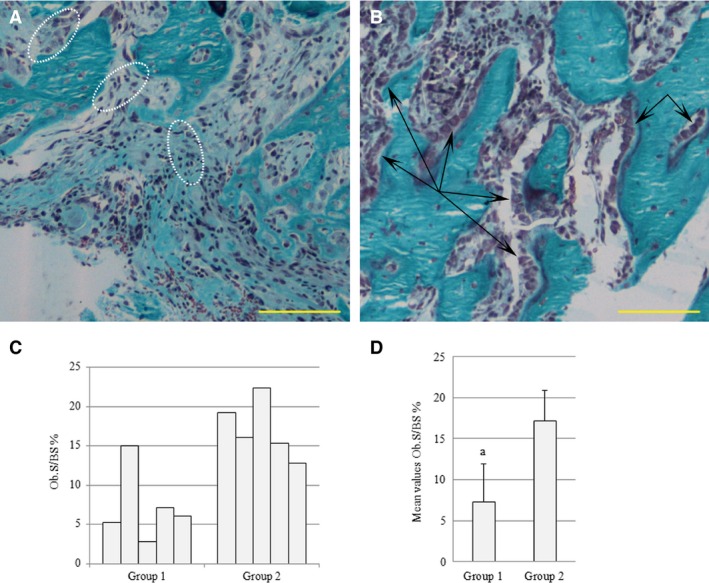
Micrographs showing new bony trabeculae inside the hole in control (A) and treated (B) animals 10 days after CBD. The cords of static osteoblasts are encircled by dotted white lines (A); the laminae of movable osteoblasts are indicated by black arrows (B). (C) Histogram reporting the values of the extent of the surface of prismatic osteoblast laminae with respect to the bone surface (Ob.S/BS) in the drilled control (group 1) and the drilled animals treated with PTH(1‐34) (group 2), 10 days after CBD. (D) Histogram reporting mean values for the same parameter; a = P < 0.05. Scale bars: (A,B) 100 μm.
Table 1.
Histomorphometric parameter (BV/TV%) of newly formed bone in healing holes in sections of femur diaphysis
| Time of sacrifice after CBD | 10 days | 28 days | 45 days |
|---|---|---|---|
| BV/TV% ‐ drilled control | 49.9 ± 8.4 | 68.7 ± 7.4 | 72.6 ± 5.3 |
| BV/TV% ‐ drilled/treated with PTH(1‐34) | 46.0 ± 4.8 | 65.1 ± 9.3 | 73.0 ± 11.2 |
All values are expressed as mean ± SD. Student's t‐test, using the stata 11.0 software (StataCorp, TX, USA) was performed to compare means of treated and control groups, according to the time of sacrifice (10, 28, 45 days).
Twenty‐eight days after CBD
Although there is the same amount of bone tissue completely filling the transcortical hole in both animal groups (Table 1), a higher amount of compact bone (at the periosteal side) than trabecular bone (at the endosteal side) is present in treated than control animals (Fig. 3). In fact, the histomorphometric parameter Ct B Ar is about 70% in treated animals and 53% in control animals, although the difference is not statistical significant. The percentage value of the total amount of calcein‐labeled newly formed bone (L B Ar) does not reveal significant differences between treated and control animals (about 9% in both groups), but the areas of calcein‐label in treated animals appear to be developed lengthwise with a linear shape (in accordance with the typical arrangement in laminae of movable osteoblasts acting during dynamic osteogenesis); in contrast, in control animals, the areas of labeled bone appear to be developed in thickness with an irregular profile (in accordance with the typical organization in cords of stationary osteoblasts acting during static osteogenesis) (Fig. 4). Concerning micro‐hardness evaluation, slight differences between control and treated groups were observed in the newly formed bone inside the holes: the micro‐hardness was higher (64 kg mm−2) in treated than control animals (59 kg mm−2), although the difference does not reach statistical significance.
Figure 3.
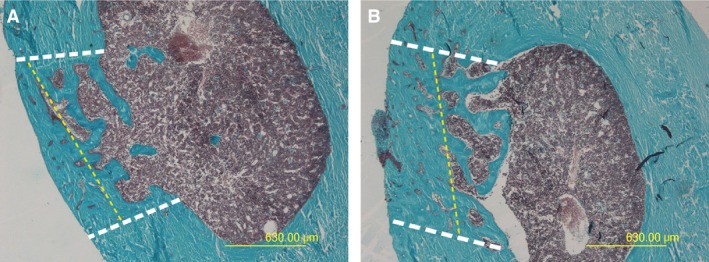
Micrographs of transverse sections of rat femurs passing through the center of the hole in drilled control (A) and drilled animals treated with PTH(1‐34) (B) 28 days after CBD. The dotted yellow lines in (A) and (B) indicate the limit between compact (left) and trabecular (right) bone (compact/trabecular bone threshold value = 30% porosity). The repaired holes are included between the dotted white lines. Note the greater amount of compact bone in drilled animals treated with PTH(1‐34) (B) with respect to drilled control (A). Scale bars: (A,B) 630 μm.
Figure 4.
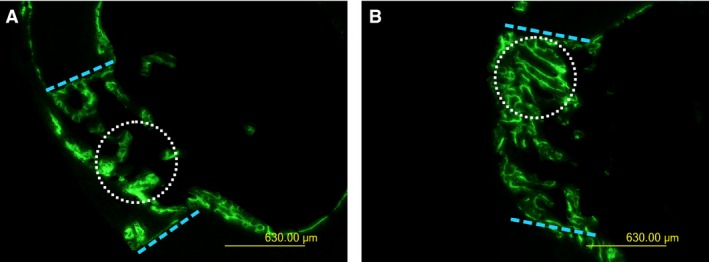
Fluorescence micrographs of transverse sections of the rat femurs passing through the center of the hole in drilled control (A) and drilled animals treated with PTH(1‐34) (B) 28 days after CBD. The dotted blue lines indicate the borders of the hole. The green label shows the newly formed bone 10 days after CBD. The dotted white circles indicate the different morphology of labeled bone areas in the two animals (irregularly shaped in A, linear in B). Scale bars: (A,B) 630 μm.
Forty‐five days after CBD
The total amount of bone tissue filling the transcortical hole (BV/TV%) did not vary in either animal group, as also observed 10 and 28 days after creation of the bone defect (Table 1). This notwithstanding, the observations of bone sections under both transmitted polarized light (Fig. 5) and SEM (Fig. 6) show a birefringence pattern (evident by means of TPL) typical of an ordered‐fibered texture (well recognizable also by SEM), abundantly evident in the treated animals compared with the control ones, thus indicating a texture made up of more ordered bone tissue instead of woven tissue in the sites of repaired lesions during administration of PTH(1‐34). Concerning micro‐hardness evaluation, trivial differences were observed between control and treated groups in the bone filling the holes.
Figure 5.
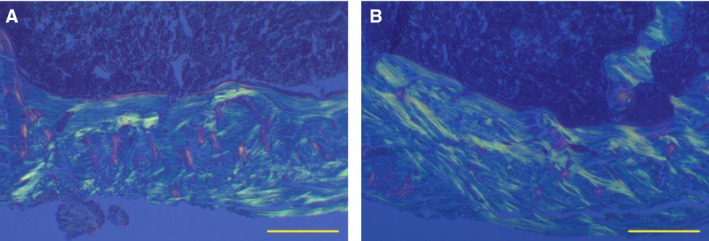
Sections from drilled control and treated animals 45 days after CBD, observed under TPL; in the central part of the hole, note in drilled control the abundance of extinguished woven bone among the primary haversian spaces (A). Note also in drilled animals treated with PTH(1‐34) the abundance of birefringent lamellar bone outlining the primary haversian osteons (B). Scale bars: (A,B) 250 μm.
Figure 6.
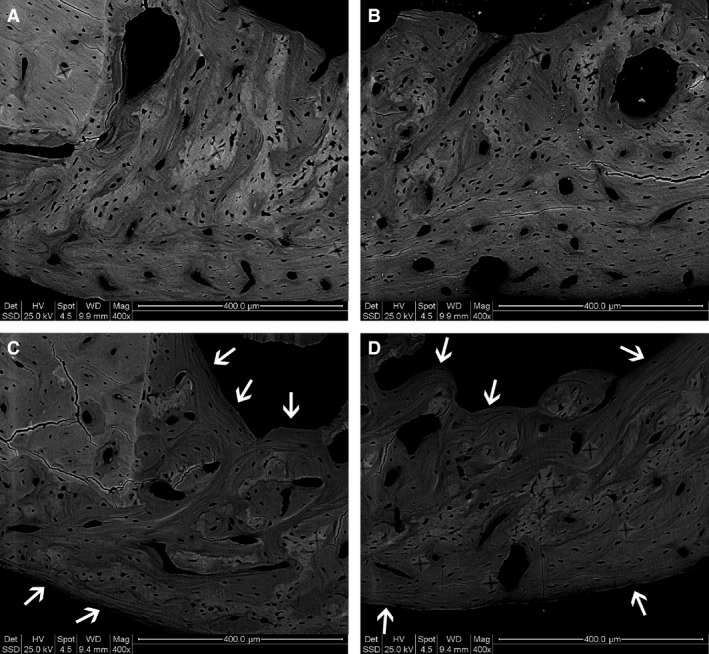
Sections from drilled control and treated animals 45 days after CBD, observed under SEM; note in drilled/treated animals (C,D) the abundance of lamellar bone (arrows) with respect to the drilled control ones (A,B). Scale bars: (A–D) 400 μm.
Serum biochemical analysis
Table 2 includes the values of parameters from sera collected at the end of the experiment. Mean values of Ca, P, OPG and BALP do not show statistically significant differences between the groups after 10, 28 and 45 days from CBD. Concerning the values of CTX and PTH (1‐84), the wide variability among the animals in each group appears significant; for these parameters, too, no differences were recorded in the mean values among all groups.
Table 2.
Serum parameters (Ca, P, OPG, BALP, CTX, PTH) at the end of the experiments
| Group | Ca, mg dL−1 | P, mg dL−1 | OPG, ng mL−1 | BALP, ng mL−1 | CTX, ng mL−1 | PTH, pg mL−1 |
|---|---|---|---|---|---|---|
| 1 | 10.35 ± 0.29 | 5.64 ± 0.48 | 0.84 ± 0.11 | 7.70 ± 0.62 | 49.42 ± 8.10 | 88.17 ± 73.89 |
| 2 | 10.03 ± 0.43 | 6.74 ± 1.45 | 0.73 ± 0.04 | 7.13 ± 0.54 | 47.64 ± 20.84 | 51.94 ± 32.34 |
| 3 | 10.16 ± 0.18 | 6.93 ± 1.29 | 0.67 ± 0.10 | 6.54 ± 1.25 | 56.31 ± 19.20 | 67.82 ± 36.17 |
| 4 | 9.87 ± 0.34 | 6.59 ± 1.26 | 0.73 ± 0.03 | 6.79 ± 0.37 | 50.06 ± 21.70 | 52.29 ± 20.30 |
| 5 | 10.17 ± 0.26 | 6.84 ± 0.41 | 0.69 ± 0.05 | 6.34 ± 1.43 | 47.59 ± 7.79 | 77.85 ± 35.81 |
| 6 | 10.25 ± 0.22 | 6.40 ± 0.42 | 0.67 ± 0.03 | 6.72 ± 0.28 | 64.7 ± 38.48 | 55.94 ± 23.98 |
Mean values ± SD of serum parameters at the end of the experiments. BALP, specific bone alkaline phosphatase, Ca, total calcium, CTX, Beta CrossLaps; OPG, osteoprotegerin, P, inorganic phosphorus, PTH, bioactive‐intact PTH(1‐84). Group 1: drilled control – 10 days; Group 2: drilled/treated with PTH(1‐34) – 10 days, Group 3: drilled control – 28 days; Group 4: drilled/treated with PTH(1‐34) – 28 days; Group 5: drilled control – 45 days; Group 6: drilled/treated with PTH(1‐34) – 45 days.
Discussion
The first point of discussion is the adequacy of the proposed animal model, in which the consecutive phases occurring during the filling of the holes are well recognizable as ongoing bone fracture repair. The most significant aspect is that, unlike the simultaneous beginning of static osteogenesis in treated and control groups, the onset of dynamic osteogenesis starts earlier in treated animals than in control animals; thus, interestingly, the compaction of the newly formed trabecular bone occurs over different times and with different patterns depending on the administration of PTH(1‐34). In fact, concerning the differentiation of dynamic osteoblasts, already 10 days after CBD, the extent of the surface of movable osteoblast laminae is significantly higher in animals with PTH(1‐34) administration than in control animals (Fig. 2). The increased osteoblast number was also demonstrated by Jilka et al. (2009) in an interesting study on the murine model after intermittent administration of parathyroid hormone. Our observations on osteoblast laminae, which differed significantly between treated and control animals, fully agree with the different morphology of bone areas labeled at 10 days by calcein and observed in treated vs. control animals sacrificed 28 days after CBD. In particular, in treated animals the labeled bone areas appear to be developed in length with a linear shape, thus resembling the typical arrangement of movable osteoblast laminae acting during dynamic osteogenesis. In contrast, in control animals the areas of labeled bone appear to be developed in thickness with an irregular profile, thus resembling the typical organization of stationary osteoblast cords acting during static osteogenesis. This critical observation strongly suggests that the crucial effect of PTH(1‐34) is to anticipate the “dynamic” formation of bone tissue with an ordered collagen texture (i.e. lamellar instead of woven‐fibered bone), widely regarded more valid from the mechanical viewpoint. Our results 28 days after CBD concerning the higher percentage of compact bone with respect to trabecular bone in treated animals is in line with the evaluation of micro‐ which showed higher values in treated animals with respect to controls; this strengthens our suggestion that PTH(1‐34) anticipates the process of dynamic osteogenesis, which produces a bone tissue more suitable for mechanical loading. Moreover, the newly formed bone of animals treated with PTH(1‐34) shows a birefringence pattern typical of a more ordered collagen fiber texture suggestive of the qualitative effect of PTH(1‐34) in improving collagen texture during bone repair. Such observations are in line with those of various authors according to whom PTH(1‐34) increases cortical bone mass by stimulating bone formation on bone surfaces (Hirano et al. 1999; Rehman et al. 2003; Iida‐Klein et al. 2007; Lindsay et al. 2007). Our results also are in accordance with the observations of Ascenzi et al. (2012) showing that the treatment of osteoporotic women with PTH(1‐34) was found to alter the heterogeneity of the orientation of collagen type I in secondary osteons in a beneficial rejuvenating way. Further, genetic and environmental changes may also impair PTH(1–34) effects on collagen type I orientation in murine models (Sage et al. 2011; Pirih et al. 2012; Ascenzi et al. 2014), thus affecting bone strength.
Analyzing the two processes of osteogenesis in detail, it should be stressed that SO is an osteo‐inductive process occurring without pre‐existing osteocytes: i.e. without mechanoreceptors (Marotti, 1996; Ferretti et al. 2002), thus making the mechanical strains ineffectual (likely to be already existing during static osteogenesis) since they cannot be sensed. In contrast, SO is responsive to cytokines such as endothelin I and/or vascular growth factors (ECGF) released by the endothelium (Streeten & Brandi, 1990; Villanueva & Nimni, 1990; Kasperk et al. 1997; Inoue et al. 2000) or by platelets (Canalis et al. 1992; Chaudhary et al. 2004), which are mitogens for osteoprogenitor cells. The primary function of SO is to provide a rigid scaffold containing osteocytes to DO‐osteoblast laminae (Ferretti et al. 2002, 2006; Palumbo et al. 2003, 2004; Marotti, 2004; Marotti et al. 2010); therefore, in DO, mechanical factors can have a crucial role in transducing mechanical stresses into biological signals. The authors suggest that this transduction could be amplified by PTH(1‐34), probably by affecting osteocyte cell‐signalling. This hypothesis appears to be in line with our recent preliminary observations (Smargiassi et al. unpubl. data) showing that in cultures of MLO‐Y4, i.e. immortalized osteocytes from murine long bones (Kato et al. 1997; Bonewald, 1999; Yang et al. 2009), the treatment (for 9 h) with PTH(1‐34) up‐regulates the expression of some genes involved in osteocyte signaling pathways with respect to untreated MLO‐Y4. This observation is also in agreement with those of Jilka et al. (2009) showing that intermittent administration of PTH(1‐34) indirectly stimulates bone formation on the surface of trabecular bone, enhancing the cell signaling pathways involved in anti‐apoptotic phenomena, which in turn lead to an increase in the osteoblast numbers. Moreover, in observing the histologic images in the cited paper of Jilka and coworkers and analyzing their results in the light of our evidences on static vs. dynamic osteogenesis (Ferretti et al. 2002), we emphasize that the conclusions obtained by Jilka et al. (‘in periosteal bone where the rate of osteoblast apoptosis is low, PTH must exert pro‐differentiating and/or pro‐survival effects on post‐mitotic pre‐osteoblasts’) refer precisely to the dynamic osteoblast laminae, since PTH(1‐34) acts on precursors of osteoblasts lining the SO‐bony trabeculae already containing osteocytes. Therefore, once again, the results of Jilka and coworkers are in line with our suggestion that PTH(1‐34) affects dynamic osteogenesis only. In agreement with our observations, a recent paper by Prideaux et al. (2015) demonstrated the effect of parathyroid hormone in inducing bone cell motility through calcium signaling. Kaback et al. (2008) suggested a mechanism for PTH(1‐34)‐mediated fracture healing possibly via Osx induction in MSCs. They showed that Osx and Runx2 are up‐regulated in marrow‐derived MSCs isolated from mice systemically treated with PTH(1‐34) and undergoing accelerated osteoblast maturation. Furthermore, systemic PTH(1‐34) treatments also accelerated fracture healing in these mice concomitantly with increased Osx expression in the PTH‐treated fracture calluses compared with controls.
As already reported by some authors, the matrix texture, strictly correlated to the bone quality (Chiang et al. 2013), strongly depends on the rate of bone remodeling: those authors report that 6‐month PTH(1‐34) treatment of patients was associated with increased bone remodeling and partial or complete healing of atypical fractures. In this context, it is important to underline that bone remodeling is exclusively due to dynamic osteogenesis, viz. exactly the osteogenic process we demonstrate to be enhanced by PTH(1‐34) administration. This is also in line with evidence provided by Ma et al. (2006) showing that PTH(1‐34) promotes the differentiation, role and lifespan of osteoblasts in the newly created bone by remodeling processes.
As far as serum parameters are concerned, it is not surprising that Ca and P levels are observed to be similar in all groups, as a consequence of the mineral homeostasis in which the skeleton, together with other systems, is involved. Furthermore BALP does not reveal differences between control and treated animals, probably because PTH(1‐34) does not affect the quantity of newly formed bone but rather the quality of bone tissue, which is, as previously mentioned, laid down in a more orderly way by the anticipated process of dynamic osteogenesis.
Conclusions
By means of the animal model proposed here, we have been able to evaluate the phase of bone healing in which PTH(1‐34) administration is crucial and mostly effective in improving the quality of skeletal repair. We demonstrate for the first time that the main effect of PTH(1‐34) concerns an earlier occurrence of dynamic osteogenesis, i.e. the deposition of best bone texture more suitable for mechanical loading. This finding could be of crucial importance in further translational clinical research in humans to define the best therapeutic strategies to be applied by orthopedic surgeons in recovering severe skeletal lesions, particularly in terms of the timing of PTH(1‐34) administration.
The authors are aware that additional studies are required to better characterize timing and related changes occurring under PTH(1‐34) treatment, concerning the expression of specific markers by bone cells engaged at the onset of bone regeneration.
Conflict of interests
All the authors who took part in this study have no conflict of interests with respect to the paper.
Animal rights and informed consent
All experiments were carried out according to the Bioethical Committee of the Italian National Institute of Health and authorized by Decrees of the Italian Ministry of Health (protocol numbers 99/2013‐B and 100/2013‐B of 12 April 2013). Animal care, maintenance and surgery were conducted in accordance with Italian law (D.L. no. 116/1992) and European legislation (EEC no. 86/609).
Author contributions
Study design: CP and MF. Study conduct: MF and FC. Data collection: FC and AS. Data analysis: MF, FC and AS. Data interpretation: CP, MF and FC. Drafting manuscript: CP and MF. Revising manuscript content: CP. Approving final version of manuscript: CP, MF, FC and AS. Fund raising: CP.
Acknowledgements
The authors thank Prof. Claudio Rovesta, Orthopedic surgeon of Policlinico of Modena, for surgical training in drilling holes, and Dr. Paola Sena and Dr. Marta Benincasa for their valuable help in animal treatments and setting iconography, respectively. This study was supported by grants of Eli‐Lilly, USA, which also provided PTH (1‐34), and by funds from ‘Fondazione di Vignola’.
References
- Ascenzi MG, Liao VP, Lee BM, et al. (2012) Parathyroid hormone treatment improves the cortical bone microstructure by improving the distribution of type I collagen in postmenopausal women with osteoporosis. J Bone Miner Res 27, 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascenzi MG, Lutz A, Du X, et al. (2014) Hyperlipidemia affects multiscale structure and strength of murine femur. J Biomech 47, 2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF (1999) Establishment and characterization of an osteocyte‐like cell line, MLO‐Y4. J Bone Miner Metab 17, 61–65. [DOI] [PubMed] [Google Scholar]
- Burr DB, Hirano T, Turner CH, et al. (2001) Intermittently administered human parathyroid hormone (1–34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res 16, 157–165. [DOI] [PubMed] [Google Scholar]
- Canalis E, Varghese S, McCarthy TL, et al. (1992) Role of platelet derived growth factor in bone cell function. Growth Regul 2, 151–155. [PubMed] [Google Scholar]
- Chaudhary LR, Hofmeister AM, Hruska KA (2004) Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone 34, 402–411. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Zebaze RMD, Ghasem‐Zadeh A, et al. (2013) Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone 52, 360–365. [DOI] [PubMed] [Google Scholar]
- Chiba S, Okada K, Lee K, et al. (2001) Molecular analysis of defect healing in rat diaphyseal bone. J Vet Med Sci 63, 603–608. [DOI] [PubMed] [Google Scholar]
- Ferretti M, Palumbo C, Marotti G (2002) Static and dynamic bone osteogenesis: two different types of bone formation. Anat Embryol 206, 21–29. [DOI] [PubMed] [Google Scholar]
- Ferretti M, Palumbo C, Bertoni L, et al. (2006) Does static precede dynamic osteogenesis in endochondral ossification as occurs in intramembranous ossification? Anat Rec A Discov Mol Cell Evol Biol 288, 1158–1162. [DOI] [PubMed] [Google Scholar]
- Hirano T, Burr DB, Turner CH, et al. (1999) Anabolic effects of human biosynthetic parathyroid hormone fragment (1–34), LY333334, on remodeling and mechanical properties of cortical bone in rabbits. J Bone Miner Res 14, 536–545. [DOI] [PubMed] [Google Scholar]
- Hock JM, Gera I (1992) Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res 7, 65–72. [DOI] [PubMed] [Google Scholar]
- Iida‐Klein A, Lu SS, Cosman F, et al. (2007) Effects of cyclic vs. daily treatment with human parathyroid hormone (1‐34) on murine bone structure and cellular activity. Bone 40, 391–398. [DOI] [PubMed] [Google Scholar]
- Inoue A, Kamiya A, Ishiji A, et al. (2000) Vasoactive peptide‐regulated gene expression during osteoblastic differentiation. J Cardiovasc Pharmacol 36, S286–S289. [DOI] [PubMed] [Google Scholar]
- Jilka RL (2007) Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40, 1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, O'Brien CA, Ali AA, et al. (2009) Intermittent PTH stimulates periosteal bone formation by actions on post‐mitotic preosteoblasts. Bone 44, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback LA, Soung do Y, Naik A, et al. (2008) Teriparatide (1‐34 human PTH) regulation of osterix during fracture repair. J Cell Biochem 105, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JM, Sarraf F, Shoichet MS, et al. (2004) Fibrin‐filled scaffolds for bone‐tissue engineering: an in vivo study. J Biomed Mater Res A 71, 162–171. [DOI] [PubMed] [Google Scholar]
- Kasperk CH, Borcsok I, Schairer HU, et al. (1997) Endothelin‐I is a potent regulator of human bone cell metabolism in vitro. Calcif Tissue Int 60, 368–374. [DOI] [PubMed] [Google Scholar]
- Kato Y, Windle JJ, Koop BA, et al. (1997) Establishment of an osteocyte‐like cell line, MLO‐Y4. J Bone Miner Res 12, 2014–2023. [DOI] [PubMed] [Google Scholar]
- Kusuzaki K, Kageyama N, Shinjo H, et al. (2000) Development of bone canaliculi during bone repair. Bone 27, 655–659. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Zhou H, Cosman F, et al. (2007) Effects of a one‐month treatment with PTH(1‐34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res 22, 495–502. [DOI] [PubMed] [Google Scholar]
- Ma YL, Zeng Q, Donley DW, et al. (2006) Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF‐II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res 21, 855–864. [DOI] [PubMed] [Google Scholar]
- Marotti G (1996) The structure of bone tissues and the cellular control of their deposition. Ital J Anat Embryol 101, 25–79. [PubMed] [Google Scholar]
- Marotti G (2004) Static and dynamic osteogenesis in the processes of bone repair. GIOT 30, S1–S5. [Google Scholar]
- Marotti G, Zaffe D, Ferretti M, et al. (2010) Static osteogenesis and dynamic osteogenesis: their relevance in dental bone implants and biomaterial osseointegration. J of Osteol and Biomat 1, 133–139. [Google Scholar]
- McDonald M, Tagil M, Morse A, et al. (2008) PTH(1‐34) treatment increases fracture callus size and strength more effectively in closed than open fractures. Bone 42, S78–S79. [Google Scholar]
- Oana L, Miclaus V, Oros D, et al. (2008) Experimental method of inducing a bone defect in rat and histological monitoring of the evolution of the healing processes. Ann Rom Soc Cell Biol 16, 111–114. [Google Scholar]
- Oswald AJ, Berg J, Milne G, et al. (2014) Teriparatide treatment of severe osteoporosis reduces the risk of vertebral fractures compared with standard care in routine clinical practice. Calcif Tissue Int 94, 176–182. [DOI] [PubMed] [Google Scholar]
- Palumbo C, Ferretti M, De Pol A (2003) Apoptosis during intramembranous ossification. J Anat 203, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo C, Ferretti M, Marotti G (2004) Osteocyte dendrogenesis in static and dynamic bone formation: an ultrastructural study. Anat Rec 278A, 474–480. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Fernandes RG, Carvalho YR, et al. (2007) Bone healing in drill hole defects in spontaneously hypertensive male and female rats’ femurs. A histological and histometric study. Arq Bras Cardiol 88, 93–97. [DOI] [PubMed] [Google Scholar]
- Pirih F, Lu J, Ye F, et al. (2012) Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res 27, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prideaux M, Dallas SL, Zhao N, et al. (2015) Parathyroid hormone induces bone cell motility and loss of mature osteocyte phenotype through L‐calcium channel dependent and independent mechanisms. PLoS ONE 10, e0125731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Q, Lang TF, Arnaud CD, et al. (2003) Daily treatment with parathyroid hormone is associated with an increase in vertebral cross‐sectional area in postmenopausal women with glucocorticoid‐induced osteoporosis. Osteoporos Int 14, 77–81. [DOI] [PubMed] [Google Scholar]
- Sage AP, Lu J, Atti E, et al. (2011) Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res 26, 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidak Z, Le Henaff C, Azzi S, et al. (2014) Low‐dose PTH increases osteoblast activity via decreased Mef2c/Sost in senescent osteopenic mice. J Endocrinol 223, 25–33. [DOI] [PubMed] [Google Scholar]
- Skripitz R, Andreassen TT, Aspenberg P (2000) Strong effect of PTH(1‐34) on regenerating bone. A time sequence study in rats. Acta Orthop Scand 71, 619–624. [DOI] [PubMed] [Google Scholar]
- Streeten EA, Brandi ML (1990) Biology of the bone endothelial cells. Bone Miner 110, 85–94. [DOI] [PubMed] [Google Scholar]
- Takao‐Kawabata R, Isogai Y, Takakura A, et al. (2015) Three‐times‐weekly administration of Teriparatide improves vertebral and peripheral bone density, microarchitecture, and mechanical properties without accelerating bone resorption in ovariectomized rats. Calcif Tissue Int 97, 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T (2006) Intermittent parathyroidhormone therapy to increase bone formation. Joint Bone Spine 73, 262–269. [DOI] [PubMed] [Google Scholar]
- Villanueva JE, Nimni ME (1990) Promotion of calvarial cell osteogenesis by endothelial cells. J Bone Miner Res 5, 733–739. [DOI] [PubMed] [Google Scholar]
- Yang W, Harris MA, Heinrich JG, et al. (2009) Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO‐Y4 osteocyte cell model. Bone 44, 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


