Figure 3.
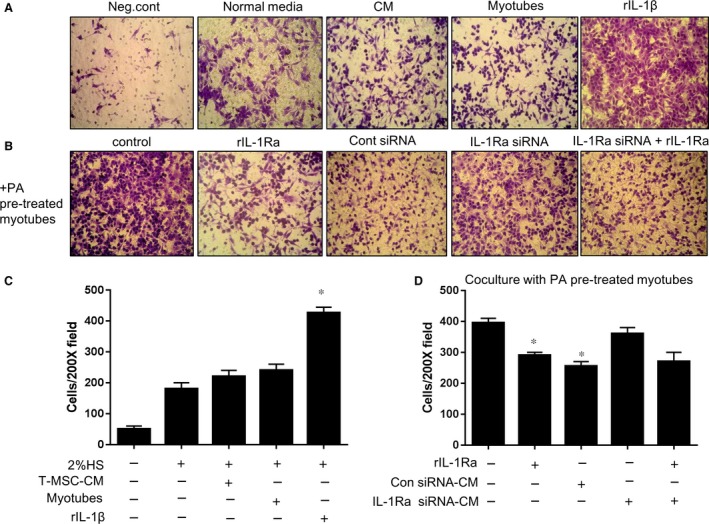
The migration of L929 fibroblasts following exposure to PA‐stimulated myotubes was abrogated by T‐CM in an IL‐1Ra‐dependent manner. (A) L929 cells were placed in the upper chamber of a transwell plate to test the ability to migrate through the membrane. In the bottom chamber, DMEM containing 2% HS was added as a normal condition. T‐CM or myotubes were placed in the bottom chamber to confirm their basal ability to recruit L929 cells. The placement of DMEM without serum or DMEM with 2% HS and 100 ng/ml of rIL‐1β in the bottom chamber were tested as the negative and positive controls, respectively. After 10 hrs, L929 cells that passed through the insert membrane and adhered to the opposite side were stained with crystal violet solution. Original magnification, 200×. (B) To confirm the effect of PA‐treated myotubes on the migration of L929 cells, myotubes were pre‐treated with 750 μM PA overnight in the bottom chamber and the media was changed after the addition of L929 cells in the upper chamber. rIL‐1Ra or T‐CM from T‐MSCs transfected with either Il‐1ra‐specific siRNA or control siRNA were added to the myotubes to test the correlation of the IL‐1β derived from myotubes and the migration of L929 cells. Exogenous rIL‐1Ra treatment (100 ng/ml) in the lower chamber containing T‐CM derived from IL‐1Ra knockdown T‐MSCs was tested as well. Original magnification, 200×. (C) L929 cells that migrated through the pores onto the lower side of the membrane were fixed, stained and counted using a phase‐contrast microscope. The data are presented as the mean ± SEM (*P < 0.05). (D) L929 cells migrated toward PA pre‐treated myotubes on the bottom in the plate were fixed, stained and counted using a phase contrast microscope. The data are presented as mean ± SEM (*P < 0.05)
