Figure 1.
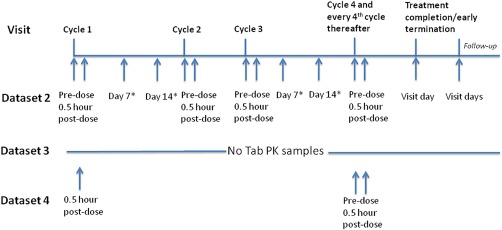
Tab PK sampling scheme for pinatuzumab vedotin and polatuzumab vedotin in the phase II study for original sampling scheme (dataset 2), all Tab PK data removed (dataset 3) and reduced Tab PK data (dataset 4). * Days are referred to as postdose of pinatuzumab vedotin or polatuzumab vedotin. Note: datasets 2, 3, and 4 are phase I data combined with all acMMAE PK data and different sampling schemes of Tab in the phase II study as shown in this figure.
