Figure 2.
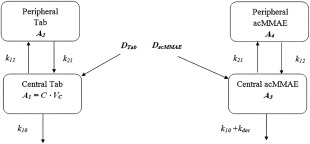
Final integrated Tab ‐ acMMAE PK model structure. A1 and A2 are the molar amounts of Tab in the central and peripheral compartments, A3 and A4 are the molar amounts of acMMAE in the central and peripheral compartments; k10 = CL/VC; k12 = Q/VC; k21 = Q/VP; CL is proteolytic clearance of the conjugate; Q is intercompartment clearance; VC is central volume and VP is peripheral volume; kdec is the deconjugation rate for acMMAE. The initial conditions are: A1(0) = DTab, A2(0) = 0, A3(0) = DacMMAE, A4(0) = 0. DTab is the dose of Tab in molar unit; DacMMAE is the dose of acMMAE in molar unit, DacMMAE = mDAR * DTab; mDAR = average drug to antibody ratio of the dosing solution (3.585 for both polatuzumab vedotin and pinatuzumab vedotin).
