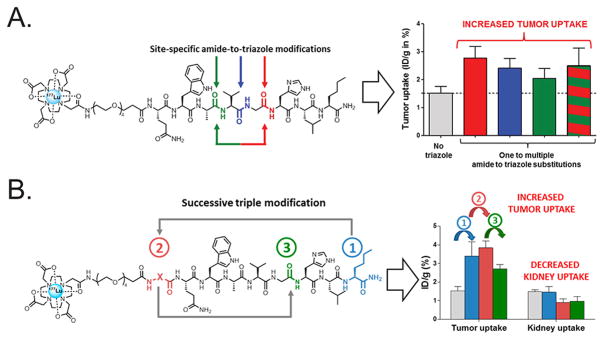
Figure 15.
(A) A series of 177Lu-labeled peptidomimetics containing 1,4-disubstituted 1,2,3-triazole moieties were shown to exhibit significantly increased proteolytic stability while retaining nanomolar affinity for GRPr. These alterations are likely responsible for ~2-fold increases in the uptake of the tracers in target-expressing PC3 xenografts compared to their triazole-lacking cousins. (B) The same methodology was successfully applied to synthesize a series of GRPr-targeting bombesin derivatives that boast high tumor-to-kidney activity concentration ratios, a critical feature in the design of therapeutic radiopharmaceuticals. Panel A was reprinted with permission from Valverde et al., copyright 2015 American Chemical Society. Panel B was reprinted with permission from reference Valverde et al., copyright 2016 American Chemical Society.