Abstract
Depression is a major public health concern with symptoms that are often poorly controlled by treatment with common antidepressants. This problem is compounded in juveniles and adolescents, because therapeutic options are limited to selective serotonin reuptake inhibitors (SSRIs). Moreover, therapeutic benefits of SSRIs are often especially limited in certain subpopulations of depressed patients, including children and carriers of low-expressing serotonin transporter (SERT) gene variants. Tricyclic antidepressants (TCAs) offer an alternative to SSRIs; however, how age and SERT expression influence antidepressant response to TCAs is not understood. We investigated the relation between antidepressant-like response to the TCA desipramine using the tail suspension test and saturation binding of [3H]nisoxetine to the norepinephrine transporter (NET), the primary target of desipramine, in juvenile (21 days postnatal [P21]), adolescent (P28), and adult (P90) wild-type (SERT+/+) mice. To model carriers of low-expressing SERT gene variants, we used mice with reduced SERT expression (SERT+/−) or lacking SERT (SERT−/−). The potency and maximal antidepressant-like effect of desipramine was greater in P21 mice than in P90 mice and was SERT genotype independent. NET expression decreased with age in the locus coeruleus and increased with age in several terminal regions (e.g., the cornu ammonis CA1 and CA3 regions of the hippocampus). Binding affinity of [3H]nisoxetine did not vary as a function of age or SERT genotype. These data show age-dependent shifts for desipramine to produce antidepressant-like effects that correlate with NET expression in the locus coeruleus and suggest that drugs with NET-blocking activity may be an effective alternative to SSRIs in juveniles.
Introduction
Depression is a major public health concern with symptoms that are often poorly controlled with commonly prescribed antidepressants. This problem is compounded in juveniles and adolescents, because there are fewer pharmacological treatment options for these patients compared with adults (Bylund and Reed, 2007). The U.S. Food and Drug Administration approved two antidepressant drugs, fluoxetine and escitalopram, for use in treating pediatric depression. Both drugs are selective serotonin reuptake inhibitors (SSRIs), which prevent serotonin (5-HT) uptake via the serotonin transporter (SERT). SSRIs can be effective treatments for adult patients suffering from depression, but these drugs often fail to relieve all depressive symptoms (Kirsch et al., 2008). The therapeutic benefit of SSRIs can be especially limited in children and in carriers of a common SERT gene variant that yields lower SERT expression (Kessler et al., 2001; Serretti et al., 2007; Bujoreanu et al., 2011). Tricyclic antidepressants (TCAs) are an alternative to SSRIs. However, in juveniles and adolescents, TCAs are not approved by the Food and Drug Administration for the treatment of depression, and they are prescribed infrequently because of serious side effects. Amitriptyline poisoning, a condition that is more dangerous in children than in adults, is one example (Paksu et al., 2014). Given that studies on the therapeutic benefit experienced by juveniles and adolescents and by carriers of low-expressing SERT gene variants from TCA treatment have reported mixed results (Hazell et al., 1995; Rajewska-Rager et al., 2008; Perlis et al., 2010; Hazell and Mirzaie, 2013), there is a need to better understand the age and SERT gene variant dependence of TCA efficacy as a first step toward developing improved antidepressants for these populations.
TCAs primarily act to block the norepinephrine transporter (NET), but some also block SERT. The ensuing increase in extracellular norepinephrine (NE) and 5-HT is thought to trigger therapeutic downstream events (Frazer, 1997). It has been suggested that a developmental delay in the noradrenergic central nervous system may limit the antidepressant potential of NET-targeting TCAs in juveniles and adolescents compared with adults (Bylund and Reed, 2007). However, other reports go against this idea. For example, in rats, innervation of noradrenergic neurons into higher brain regions, such as the cortex, reach adult-like morphology by postnatal day (P) 7 (Loizou, 1972; Lauder and Bloom, 1974; Coyle, 1977; Thomas et al., 1995). Noradrenergic receptors reach adult levels by P14–P21, and norepinephrine content reaches adult levels by P14–P42 (Loizou and Salt, 1970; Konkol et al., 1978; Morris et al., 1980; for review, see Murrin et al., 2007). In juvenile and adolescent rats, NET expression is reported to be greater or equivalent to that in adults in numerous brain regions (Moll et al., 2000; Sanders et al., 2005). These findings suggest that NET-selective TCAs, such as desipramine (DMI), should produce antidepressant-like effects in juvenile (P21) and adolescent (P28) rodents, because the noradrenergic system is relatively established at these ages.
However, there is a paucity of research investigating antidepressant-like effects of TCAs in juvenile and adolescent rodents. In rats, the TCAs imipramine and DMI have been reported to be less effective in producing antidepressant-like effects in the forced swim test (FST) in juveniles than in adults (Reed et al., 2008). In contrast, we found that 32 mg/kg DMI produced equivalent antidepressant-like effects in mice aged P21, P28, and P64–P90 (young adult) in the tail suspension test (TST) (Mitchell et al., 2013). These behavioral results were paralleled by [3H]nisoxetine binding in whole hippocampal homogenates, which revealed no difference in NET expression among these ages. Although these studies are informative, they provide no information on how age may affect the potency or maximal effect of DMI to produce antidepressant-like effects in the TST. [3H]Nisoxetine binding assays in hippocampal homogenates also lack the ability to discriminate potential differences in NET expression among hippocampal subregions as a function of age and do not provide information about other brain regions that may be important in mediating antidepressant-like effects.
Determining the potency and maximal effect of DMI to produce antidepressant-like effects in the TST, as well as establishing the ontogeny of NET expression throughout early postnatal development, could help to identify age-dependent mechanisms that may limit the therapeutic benefit of antidepressants. Here, we examined the dose-response relationship for DMI to produce antidepressant-like effects in P21, P28, and P90 SERT-deficient mice, and we quantified NET expression in a number of brain regions using autoradiography with [3H]nisoxetine, a NET-selective ligand. SERT-deficient mice were included in this study to assess the effects of constitutive reduction in SERT expression, which occurs in humans carrying low-expressing gene variants of SERT, on antidepressant-like effects of DMI and on NET expression and affinity.
Materials and Methods
Animals
Naïve male and female SERT wild-type (SERT+/+), heterozygote (SERT+/−), or homozygote knockout (SERT−/−) mice (backcrossed to C57BL/6J for >10 generations) were used for all experiments. Dr. Dennis Murphy (National Institute of Mental Health, Bethesda, MD) provided mice to found the colony. Animals were bred by crossing male and female SERT+/− mice, and SERT genotypes were identified as previously described (Bengel et al., 1998). Mice were aged P21 (juvenile), P28 (adolescent), and P90–P100 (adult) (Spear, 2000) for all experiments. Animals were housed in a temperature-controlled (24°C) vivarium maintained on a 12-hour/12-hour light/dark cycle (lights on at 7:00 AM) in plastic cages (29 cm × 18 cm × 13 cm) containing rodent bedding (Sani-chips; Harlan Teklad, Madison, WI) with free access to food (irradiated rodent sterilizable diet; Harlan Teklad) and water. Weaning occurred at P28, after which mice were housed with five of their same-sex peers. To avoid possible confounds of treatment effects with litter effects, no more than one mouse from a given litter was assigned to a particular treatment condition. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf) and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
TST
TST experiments were conducted as originally described by Steru et al. (1985) with minor modifications (described in Mitchell et al., 2015). In brief, mice were moved from a housing facility to a testing room and then given a 1- to 2-hour acclimation period prior to TST. Experiments were conducted in the afternoon, between 12:00 PM and 5:00 PM. Mice received an intraperitoneal injection of saline 1 hour prior to testing, followed by an intraperitoneal injection of either DMI (3.2, 10, or 32 mg/kg) or saline vehicle (control condition) 30 minutes prior to testing. This two-injection protocol was used to be consistent with previous studies from this laboratory (Baganz et al., 2008; Horton et al., 2013) and with future studies that will examine the effects of drug combinations on immobility time in the TST. Immediately before testing, the distal portion of the tail was secured to a flat aluminum bar (2 × 0.3 × 10 cm) using adhesive tape. A hole on the opposite end of the bar was secured to a hook in a visually isolated white box (40 × 40 × 40 cm). Each mouse was suspended for 6 minutes while a digital video camera recorded its ventral surface. Immobility was defined as the absence of active movements and included passive swaying. Immobility time was scored in seconds by observers blind to the randomly assigned treatment conditions. Mice were tested only once.
Drugs
DMI (desmethylimipramine) hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in physiologic saline and injected intraperitoneally at doses expressed as salt weight per kilogram body weight. The injection volume was 10 ml/kg.
Autoradiography
NET density in the mouse brain was assessed by quantitative autoradiography using the NET-selective ligand [3H]nisoxetine and methods adapted from Tejani-Butt (1992). Mice were euthanized by decapitation and brains were flash frozen on powdered dry ice before being stored at −80°C. Before sectioning, brains were brought to −20°C in a cryostat (Leica CM 1850; Meyer Instruments, Houston, TX). Coronal sections (20 µm) were collected at the level of plate 12 (prefrontal cortex), plate 47 (hippocampal subregions including the cornu ammonis regions CA1, CA2, and CA3 and the dentate gyrus), the amygdala, plate 64 (raphe nuclei), and plate 76 (locus coeruleus) according to the Paxinos and Franklin (1997) mouse brain atlas. Sections were thaw mounted onto gelatin-coated microscope slides. Tissue-mounted slides were vacuum desiccated for 18–24 hours at 4°C before the slides were stored at −80°C. Brain tissue was stored at −80°C for 2–4 weeks, and every experiment contained brain tissue from all groups, including P21, P28, and P90 SERT+/+, SERT+/−, and SERT−/− male and female mice. Before incubation, sections on slides were thawed for 1 hour in a vacuum desiccator at 4°C to remove excess moisture and maximize brain tissue adherence to the slides. The slides were then preincubated for 1 hour in a wash buffer solution (43 mM Tris-HCl, 124 mM NaCl, and 4.3 mM KCl, pH 7.4) at room temperature (approximately 24°C) to remove endogenous ligands bound to NET. Incubation was carried out in slide mailers (VWR International, Radnor, PA) filled with 10 ml ice-cold reaction buffer (50 mM Tris-HCl, 300 mM NaCl, and 5 mM KCl, pH 7.4) containing [3H]nisoxetine at concentrations of 0.3, 1, 3, 10, or 30 nM for 4 hours. Each concentration of [3H]nisoxetine was incubated with three brain sections per animal per brain region. Nonspecific binding was defined by 2.5 mM mazindol (Pfizer, Groton, CT) and was approximately 9%–50% total binding in low binding regions (i.e., CA1) and approximately 9%–22% total binding in high binding regions (i.e., locus coeruleus). The incubation was terminated by three 5-minute washes in wash buffer solution at 4°C, followed by a 5-second dip in deionized water at 4°C. Slides were dried on a slide warmer for 20 minutes. [3H]Nisoxetine-labeled sections were exposed to Carestream Biomax MR film for 6 weeks, along with tritium standards (American Radiolabeled Chemicals, St. Louis, MO). Films were developed in a film processor (AFP Imaging, Elmsford, NY). A digital imaging system that included a 12-bit digital camera (CFW-1612M; Scion Corp., Frederick, MD), Nikon Lens, Northern Lights Illuminator, and Kaiser RS1 copy stand (all from InterFocus Imaging Ltd., Linton, UK) was used to capture autoradiogram images. Autoradiograms were calibrated and measured using ImageJ public access shareware (National Institutes of Health, Bethesda, MD; https://imagej.nih.gov/ij/download.html) on a MacBook computer (OS 10; Apple, Cupertino, CA). Additional brain sections were stained with thionine (FD NeuroTechologies, Inc., Columbia, MD) to verify tissue integrity and neuroanatomical brain regions quantified (Figs. 2B and 3B).
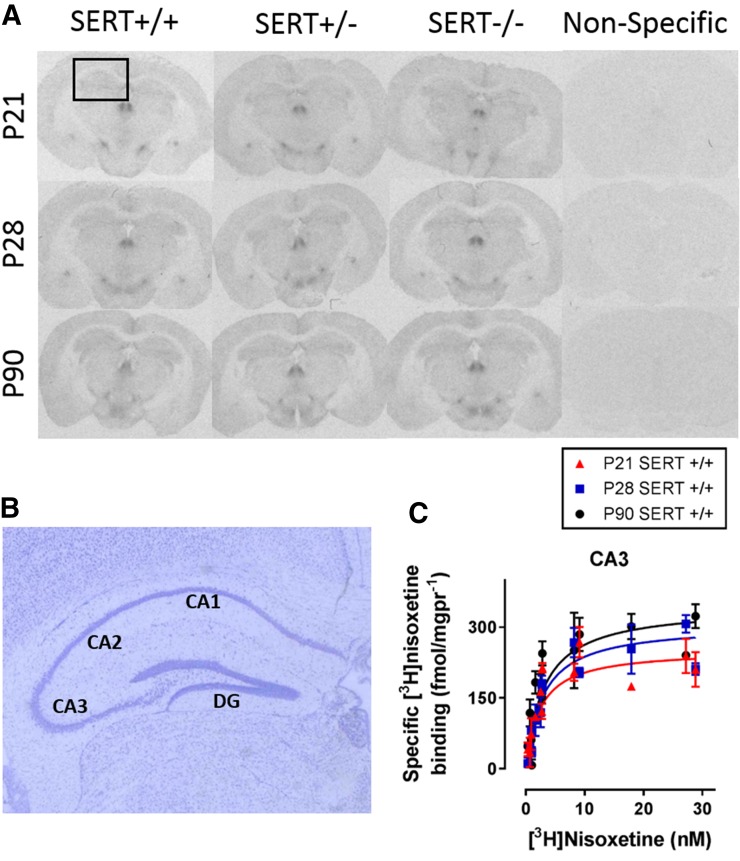
Fig. 2.
Specific [3H]nisoxetine binding to NET in hippocampal regions as a function of age and SERT genotype. Brain sections from P21, P28, and P90 SERT-deficient mice incubated with the NET-specific ligand [3H]nisoxetine. Nonspecific binding was defined by mazindol (2.5 mM). (A) Representative coronal sections at the level of plate 47 (Paxinos and Franklin, 1997) in SERT+/+, SERT+/−, and SERT−/− mice aged P21, P28, or P90. The boxed area in (A) is enlarged in (B), which shows representative thionine-stained brain sections labeled with hippocampal regions quantified, which include the CA1, CA2, and CA3 regions and the dentate gyrus (DG). (C) Example of saturation binding isotherms used to calculate Bmax and Kd values. Curves include specific [3H]nisoxetine binding values for the CA3 of P21, P28, and P90 SERT+/+ mice. There was no main effect of sex on Bmax or Kd values, so male and female data are pooled (P > 0.05). Bmax values are summarized in Fig. 4. There were no significant differences in Kd among ages or between SERT+/+ and SERT+/− mice. Sample sizes of mice per group were as follows: SERT+/+, n = 5–9 (4 males and 3–5 females, pooled); SERT+/−, n = 6–10 (3–5 males and 2–4 females, pooled); and SERT−/−, n = 4–7 (2–4 males and 2–4 females, pooled). See Table 2 and Fig. 4 for a summary of data.
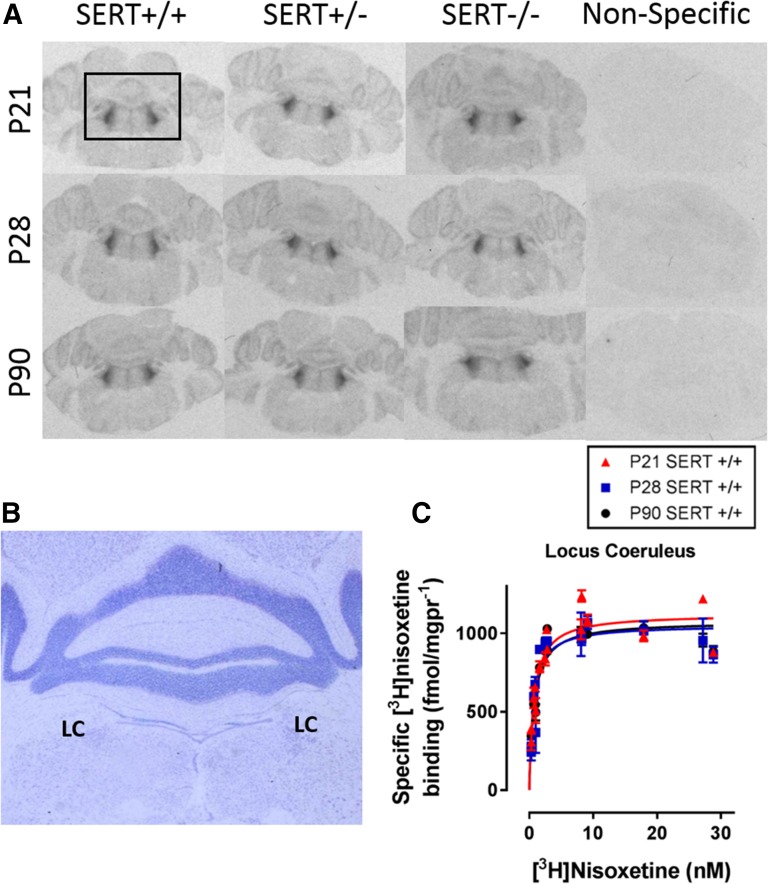
Fig. 3.
Specific [3H]nisoxetine binding to NET in the locus coeruleus as a function of age and SERT genotype. Brain sections from P21, P28, and P90 mice were incubated with increasing concentrations of [3H]nisoxetine. Nonspecific binding was defined by mazindol (2.5µM). (A) Representative coronal sections at the level of plate 76 (Paxinos and Franklin, 1997) in SERT+/+, SERT+/−, and SERT−/− mice aged P21, P28, or P90. The boxed area in (A) is enlarged in (B), which shows a representative thionine-stained brain sections including the locus coeruleus (LC). (C) Example of saturation binding isotherms used to calculate Bmax and Kd values. Curves include specific [3H]nisoxetine binding values for the LC of P21, P28, and P90 SERT+/+ mice. Bmax values for this region are summarized in Fig. 4. There was no main effect of sex on Bmax or Kd values, so male and female data are pooled (P > 0.05). Sample sizes of mice per group were as follows: SERT+/+, n = 5–9 (4 males and 3–5 females, pooled); SERT+/−, n = 6–10 (3–5 males and 2–4 females, pooled); and SERT−/−, n = 4–7 (2–4 males and 2–4 females, pooled). See Table 2 and Fig. 4 for a summary of data.
Data Analysis
Statistical analyses were performed using GraphPad Prism (version 6.0; GraphPad Inc., La Jolla, CA) and NCSS 2007 (NCSS, Kaysville, UT) software.
TST.
Dose-response curves were generated by administering 3.2, 10, or 32 mg/kg DMI or saline vehicle in juvenile, adolescent, or adult mice. Under vehicle conditions, we previously found that time spent immobile varies by age and SERT genotype (Mitchell et al., 2013, 2015, 2016). Because such differences were also apparent in our study (see vehicle data in Fig. 1, A–C), we expressed data as total immobility in seconds (Fig. 1, A–C) and then replotted these data as the percentage from the saline vehicle control (Fig. 1, D–F). Within genotype, TST data were analyzed by a two-factor analysis of variance (ANOVA) (age, drug dose) followed by Dunnett and Tukey multiple comparisons tests. Sample sizes for TST data included the following per data point: 9–14 SERT+/+ males and 10–20 SERT+/+ females, 8–10 SERT+/− males and 16–20 SERT+/− females, and 8–10 SERT−/− males and 9–14 SERT−/− females. A multifactor ANOVA showed neither a main effect of sex nor any interaction of sex with other factors (P = 0.51 and P ≥ 0.10, respectively), with the exception of a sex × genotype interaction (P = 0.008); however, multiple comparisons failed to show significant sex differences for each of the genotypes. Thus, data for males and females were combined. Maximal effects (Emax) and half-maximally effective dose (ED50) values were derived from data in Fig. 1, D–F, and are summarized in Table 1. Emax was defined as the greatest observed percent change of immobility from the saline control. Emax values were analyzed by two-factor ANOVA (age, SERT genotype) followed by the Tukey multiple comparisons test (Table 1). ED50 values were calculated using methods detailed in Koek et al. (2009). Briefly, the linear portion of the dose-response curves was analyzed by log-linear regression [effect = slope × log(dose) + intercept] of data from individual subjects. All data are expressed as means ± S.E.M. except for ED50 values, which are expressed as the mean. P < 0.05 was considered statistically significant.
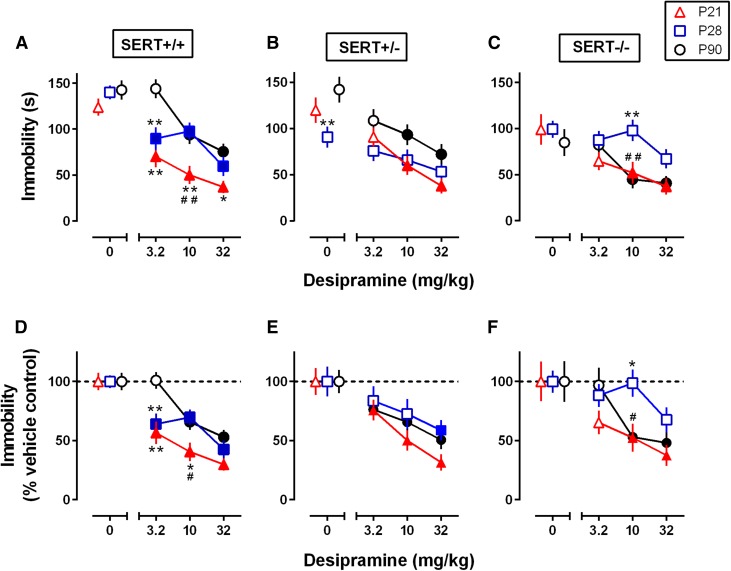
Fig. 1.
Influence of age and SERT genotype on the antidepressant-like effect of DMI. (A–C) Dose-dependent reductions in immobility time in the TST in P21, P28, and P90 SERT+/+ mice (A), SERT+/− mice (B), and SERT−/− mice (C). (D) Data from (A) expressed as a percentage of vehicle control. (E) Data from (B) expressed as a percentage of vehicle control. (F) Data from (C) expressed as a percentage of vehicle control. Data obtained in males and females are pooled, because a multifactor ANOVA (sex, genotype, DMI) showed no main effect or interaction of sex with other factors (P > 0.05), with one exception between sex and genotype (P < 0.01); however, multiple comparisons failed to show significant sex differences for each genotype (P > 0.05). Data are means ± S.E.M. Filled symbols represent data points that are significantly different from the SERT genotype– and age-matched vehicle control as determined by the Dunnett post hoc multiple comparisons test after a two-factor ANOVA. *P < 0.05 (significant difference from SERT genotype–matched P90); **P < 0.01 (significant difference from SERT genotype–matched P90); #P < 0.05 (significant difference from SERT genotype–matched P28 with the Tukey post hoc multiple comparisons test after a two-factor ANOVA); ##P < 0.01 (significant difference from SERT genotype–matched P28 with the Tukey post hoc multiple comparisons test after a two-factor ANOVA). Sample sizes per data point were as follows: SERT+/+, n = 21–31 (9–14 males and 10–20 females, pooled); SERT+/−, n = 16–20 (8–10 males and 8–12 females, pooled); and SERT−/−, n = 18–23 (8–10 males and 9–14 females, pooled).
TABLE 1.
Influence of age and SERT genotype on the potency (ED50) and maximal effect (Emax) of DMI to reduce immobility time in the TST
Data are expressed as means ± S.E.M. Values were calculated from data shown in Fig. 1, D–F, and Table 1.
| Age | SERT | ED50 | Emax |
|---|---|---|---|
| mg/kg | %Δ Control | ||
| P21 | +/+ | 7.5*,** | 70.1 ± 5.4* |
| +/− | 17.4** | 68.5 ± 7.0 | |
| −/− | 17.1** | 62.4 ± 8.9** | |
| P28 | +/+ | 23.7* | 57.5 ± 10.6 |
| +/− | 36.3 | 41.3 ± 8.6 | |
| −/− | 45.2*** | 32.3 ± 9.2 | |
| P90 | +/+ | 37.3 | 47.0 ± 6.1 |
| +/− | 29.1 | 47.3 ± 7.7 | |
| −/− | 30.5 | 51.9 ± 9.2 |
Sample sizes per data point were as follows: SERT+/+, n = 21–31 (9–14 males and 10–20 females, pooled); SERT+/−, n = 16–20 (8–10 males and 8–12 females, pooled); and SERT−/−, n = 18–23 (8–10 males and 9–14 females, pooled). For ED50 values, comparisons were made by the F ratio test to compare intercepts. For Emax values, comparisons made by the Tukey post hoc multiple comparisons test after a two-factor ANOVA. Because there were no statistically significant sex differences, data for male and female mice were pooled.
P < 0.05 (difference from SERT genotype–matched P90); **P < 0.05 (difference from SERT genotype–matched P28); ***P < 0.05 (difference from age-matched SERT+/+ group).
Quantitative Autoradiography.
[3H]Nisoxetine binding densities were measured from autoradiograms and analyzed using methods described in Mitchell et al. (2016). In brief, nonspecific binding was fit to an unweighted linear regression and subtracted from total [3H]nisoxetine to give specific binding. Unweighted nonlinear regression was used to analyze [3H]nisoxetine-specific binding data. Saturation binding isotherms were fitted according to the following one-site model to calculate maximal specific [3H]nisoxetine binding (Bmax) and affinity (Kd) values: Y = Bmax × X/(Kd + X) (Figs. 2 and 3). Bmax and Kd values were analyzed with a two-factor (age, genotype) ANOVA (Fig. 4; Table 2). There were no statistically significant effects of sex (P ≥ 0.09) or sex and age interactions (P ≥ 0.17) for [3H]nisoxetine Bmax or Kd values within genotype- and brain region–matched groups; thus, data from both sexes were combined. Two-factor ANOVA (age, SERT genotype) (Fig. 4) followed by the Dunnett post hoc test for multiple comparisons was used to analyze mean Bmax and Kd values. All data are expressed as means ± S.E.M. P < 0.05 was considered statistically significant.
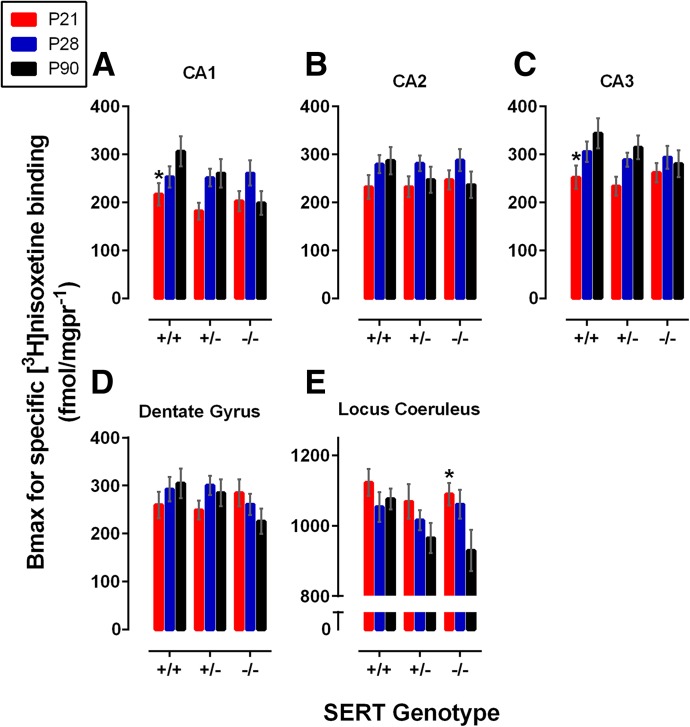
Fig. 4.
Bmax for [3H]nisoxetine binding to SERT in SERT+/+ and SERT+/− mice aged P21, P28, and P90. Bmax values from P21, P28, and P90 SERT+/+, SERT+/−, and SERT−/− mice in the CA1 region (A), CA2 region (B), CA3 region (C), dentate gyrus (D), and locus coeruleus (E) were determined from one-site curve fits as described in the Materials and Methods. Data are means ± S.E.M. *P < 0.05 (significant difference from SERT genotype–matched P90, Dunnett post hoc multiple comparisons test after a two-factor ANOVA for age and SERT genotype). Data are means ± S.E.M. pooled from male and female mice. Sample sizes of mice per group were as follows: SERT+/+, n = 5–9 (4 males and 3–5 females, pooled); SERT+/−, n = 6–10 (3–5 males and 2–4 females, pooled); and SERT−/−, n = 4–7 (2–4 males and 2–4 females, pooled).
TABLE 2.
Summary of Bmax values for specific [3H]nisoxetine binding in SERT+/+, SERT+/−, and SERT−/− mice
Data are expressed as means ± S.E.M. in femtomoles per milligram protein.
| Genotype | P21 | P28 | P90 |
|---|---|---|---|
| Prefrontal cortex | |||
| SERT+/+ | 228 ± 23* | 275 ± 26 | 314 ± 36 |
| SERT+/− | 229 ± 23 | 251 ± 21 | 290 ± 30 |
| SERT−/− | 206 ± 19 | 271 ± 35 | 225 ± 26 |
| Amygdala | |||
| SERT+/+ | 193 ± 27 | 137 ± 14* | 233 ± 29 |
| SERT+/− | 150 ± 29 | 165 ± 14 | 191 ± 24 |
| SERT−/− | 157 ± 24 | 146 ± 20 | 155 ± 21 |
| Dorsal raphe | |||
| SERT+/+ | 619 ± 40 | 551 ± 35 | 539 ± 35 |
| SERT+/− | 560 ± 57 | 562 ± 31 | 578 ± 27 |
| SERT−/− | 565 ± 20 | 543 ± 24 | 536 ± 36 |
Sample sizes of mice per group were as follows: SERT+/+, n = 5–9 mice per group (4 males and 3–5 females, pooled); SERT+/−, n = 6–10 (3–5 males and 2–4 females, pooled); and SERT−/−, n = 4–7 (2–4 males and 2–4 females, pooled).
P < 0.05 (different from SERT genotype–matched P90 group; Dunnett multiple comparisons test after two-factor ANOVA).
Correlations.
As shown in Fig. 5, Pearson correlation was used to examine the relation between the Emax for DMI to reduce immobility time in the TST and maximal specific [3H]nisoxetine binding, as a function of age. Within each brain region, SERT genotype did not significantly influence the relation between the Emax for DMI and maximal specific [3H]nisoxetine binding (P ≥ 0.15; data not shown) so data for SERT+/+, SERT+/−, and SERT−/− were best fit with a single line (Fig. 5). All data are expressed as means ± S.E.M.
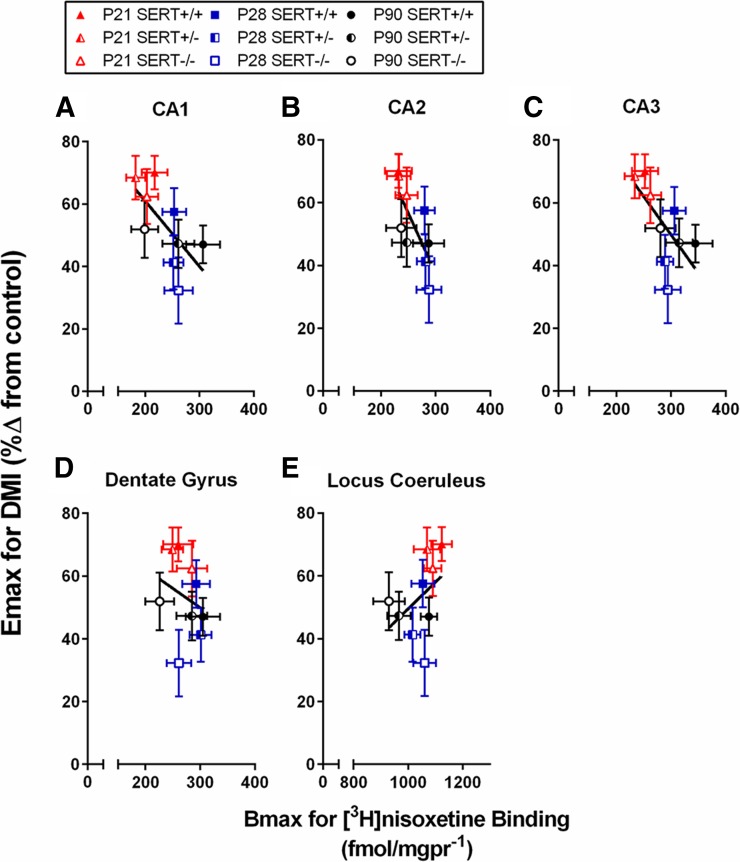
Fig. 5.
Relationship between Emax values for DMI to produce antidepressant-like effects in the TST and Bmax values for specific [3H]nisoxetine binding in the locus coeruleus as a function of age and SERT genotype. The CA1 region (A), CA2 region (B), CA3 region (C), dentate gyrus (D), and locus coeruleus (E) are shown. Relationship between Emax and Bmax did not vary by SERT genotype; thus, one line was used to fit data regardless of genotype. Data are taken from Fig. 1, D–F (per data point: SERT+/+, n = 21–31; SERT+/−, n = 16–20; and SERT−/−, n = 18–23), and Fig. 4 (per age group: SERT+/+, n = 5–9; SERT+/−, n = 6–10; and SERT−/−, n = 4–7). Data are means ± S.E.M. (male and female data are pooled).
Results
The Potency of DMI to Reduce Immobility Time in the TST Depends on Age and SERT Genotype, Whereas Its Maximal Effect Depends on Age Only
Clinical studies have shown that therapeutic potential of SSRIs is limited in children (Kirsch et al., 2008). Similarly, we previously found that the antidepressant-like response to SSRIs in juvenile mice is limited compared with adult mice (Mitchell et al., 2013, 2016). As an alternative to SSRIs, we began our study by evaluating the antidepressant-like response to DMI, a potent NET blocker, in SERT+/+ P21, P28, and P90 mice. Using the TST, we found that DMI reduced immobility time in all age groups [F(3,276) = 35.06, P < 0.01], and younger mice spent overall less time immobile than adult mice [F(2,276) = 19.73, P < 0.01] (Fig. 1A). An interaction between age and DMI dose showed that younger mice are more sensitive to the anti-immobility effects of DMI than adults [F(6,276) = 2.34, P = 0.03]. Post hoc analysis revealed that immobility time was significantly lower in P21 mice than in P28 and P90 mice after administration of 10 mg/kg DMI (P < 0.01), and immobility time was lower in P21 mice than in P90 mice after administration of 3.2 and 32 mg/kg DMI (P < 0.01 and P = 0.012, respectively). Likewise, immobility time at 3.2 mg/kg DMI was significantly lower in P28 mice than in P90 mice (P < 0.01). Furthermore, 3.2 mg/kg DMI was the lowest effective dose in P21 and P28 mice, and 10 mg/kg was the lowest effective dose in P90 mice (P < 0.01). Taken together, the anti-immobility effects of DMI appear to be greater in younger mice than adults.
Clinical studies have also found that the antidepressant effects of SSRIs are limited in individuals with a SERT gene polymorphism that yields a reduction in SERT expression/function (Serretti et al., 2007). We used SERT+/− mice, which express 50% less SERT than SERT+/+ mice, to evaluate the antidepressant-like potential of NET blockade in individuals with reduced SERT expression/function. DMI reduced immobility time in the TST [F(3,212) = 16.58, P < 0.01] (Fig. 1B), and younger mice spent overall less time immobile in the TST than adult mice [F(2,212) = 9.34, P < 0.01]. In contrast with SERT+/+ mice, no interaction was found between age and DMI drug dose in SERT+/− mice [F(6,212) = 0.81, P = 0.57]. Post hoc analysis showed that immobility time in the TST was lower in vehicle treated P28 mice than vehicle treated P90 mice (P < 0.01). The lowest effective dose for P21 and P90 mice was 10 mg/kg DMI (P < 0.01). Although no dose of DMI significantly reduced immobility time in P28 mice, immobility times after 32 mg/kg DMI trended to be lower than immobility times after vehicle treatment (P = 0.087). These data suggest that the antidepressant-like effects of DMI are not greater in younger SERT+/− mice than in adult mice; however, variation in basal immobility (saline vehicle control) as seen with P28 SERT+/− mice may limit the interpretation of our findings.
Although DMI is a potent NET blocker, it does have affinity for SERT. The antidepressant-like effect of DMI in SERT−/− mice was evaluated to investigate the proportion of the antidepressant-like response that was SERT dependent in P21, P28, and P90 mice. Immobility time in the TST was reduced after DMI administration [F(3,217) = 9.00, P < 0.01], although no dose of DMI significantly lowered immobility time in P28 mice (P = 0.13). A main effect of age was also found [F(2,217) = 6.14, P < 0.01] (Fig. 1C). No interaction between age and drug dose was found [F(6,217) = 0.29, P = 0.94]. Immobility time was lower in P21 and P90 mice than in P28 mice after administration of 10 mg/kg DMI (P = 0.015, P < 0.01). The lowest effective dose for P21 and P90 mice was 10 mg/kg DMI (P < 0.01 and P = 0.04, respectively). These data suggest that NET blockade is primarily responsible for the antidepressant-like effects of DMI in the TST, with the possible exception of P28 SERT−/− mice, in which (as for SERT+/− mice) no dose of DMI significantly reduced immobility time (Fig. 1C).
It should be noted that basal immobility time in the TST was lower in SERT−/− mice (Fig. 1C) compared with SERT+/+ mice (Fig. 1A), and P28 SERT+/− mice showed less basal immobility compared with P90 SERT+/− mice (Fig. 1B). To account for age- and SERT genotype–related variations in basal immobility time, data were normalized to a percentage of vehicle control (Fig. 1, D–F). The analysis of the normalized SERT+/+ data was similar to the analysis of raw SERT+/+ data (in seconds) with minor exceptions. DMI reduced immobility time in all SERT+/+ age groups [F(3,276) = 36.23, P < 0.01], and a main effect of mouse age was found [F(2,276) = 10.36, P < 0.01] (Fig. 1D). Younger mice showed greater sensitivity to the anti-immobility effects of DMI in the TST than adult mice [F(6,276) = 2.58, P = 0.02]. Immobility time was significantly lower in P21 SERT+/+ mice than in P90 mice after 3.2 mg/kg (P < 0.01) and 10 mg/kg DMI (P = 0.04). Immobility time was lower in SERT+/+ P28 mice than in P90 mice after 3.2 mg/kg DMI (P < 0.01). The lowest effective dose for DMI was 3.2 mg/kg in SERT+/+ mice aged P21 and P28 (P < 0.01), and the lowest effective dose for P90 mice was 10 mg/kg (P < 0.01).
Analysis of normalized SERT+/− data showed that DMI reduced immobility time in the TST [F(3,212) = 15.7, P < 0.01], with no main effect of age [F(2,212) = 2.091, P = 0.13] (Fig. 1E). DMI reduced immobility time in all age groups, and it did so equally across all age groups [F(6,212) = 0.46, P = 0.84]. No differences among age groups were found. The lowest effective dose for P21 and P90 SERT+/− mice was 3.2 mg/kg DMI (P < 0.01 and P = 0.03, respectively). The lowest effective dose for P28 SERT+/− mice was 32 mg/kg DMI (P = 0.02). By normalizing SERT+/− data, we found that DMI reduced immobility in all age groups, including P28 SERT+/− mice.
Normalized data for SERT−/− mice showed a main effect of DMI [F(3,217) = 9.01, P < 0.01] and age [F(2,217) = 4.20, P = 0.02] (Fig. 1F). Like SERT+/− mice, there was no interaction between age and drug dose [F(6,217) = 0.27, P = 0.95]. After 10 mg/kg DMI, immobility time was significantly lower in P21 and P90 SERT−/− mice than in P28 mice (P = 0.02 and P = 0.03, respectively). The lowest effective dose for P21 and P90 SERT−/− mice was 10 mg/kg (P = 0.02); in addition, no dose significantly reduced immobility time in P28 mice, including 32 mg/kg DMI (P = 0.17).
To investigate a possible interaction between postnatal age and SERT genotype on the antidepressant-like response to DMI, we compared the potency (ED50) and maximal effect (Emax) of DMI at different ages and in different genotypes (Table 1). With the exception of P28 SERT+/− and SERT−/− mice, ED50 values for DMI were lower in younger mice than in adults. ED50 values for DMI varied as a function of age in SERT+/+, SERT+/−, and SERT−/− mice [F(2,205) = 12.57, P < 0.01; F(2,163) = 3.22, P = 0.04; and F(2,149) = 3.38, P = 0.04, respectively]. In SERT+/+ mice, the ED50 value was lower in P21 mice than in P28 mice and P90 mice [F(1,125) = 6.75, P = 0.02; and F(1,143) = 25.0, P < 0.01], and P28 mice had a lower ED50 value than P90 mice [F(1,143) = 5.2, P = 0.02]. In SERT+/− mice, ED50 values were lower in P21 mice than in P28 mice [F(1,105) = 5.7, P = 0.02]. In SERT−/− mice, ED50 values were lower in P21 mice than in P28 mice [F(1,96) = 9.33, P < 0.01]. Among SERT genotype comparisons showed an effect of SERT genotype in P28 mice [F(2,167) = 5.50, P < 0.01]. P28 SERT+/+ mice showed a lower ED50 than P28 SERT−/− mice [F(1,116) = 12.25, P < 0.01]. SERT genotype had no significant main effect on ED50 values in P21 or P90 mice [F(2,177) = 1.29, P = 0.28; and F(2,193) = 0.82, P = 0.44]. In P28 mice, an effect of SERT genotype was found [F(2,167) = 5.50, P < 0.01]. P28 SERT+/+ mice had a lower ED50 value than P28 SERT−/− mice [F(1,116) = 12.25, P < 0.01].
The Emax for DMI to reduce immobility time in the TST was significantly greater in young mice than in adults [F(2,178) = 7.06, P < 0.01]. P21 SERT+/+ mice showed a significantly greater Emax than P90 SERT+/+ mice (P = 0.046). In addition, the Emax for DMI in P21 SERT−/− mice was greater than P28 SERT−/− mice (P = 0.02). SERT genotype did not affect the Emax for DMI to reduce immobility time [F(2,178) = 1.043, P = 0.35], and SERT genotype did not interact with the effects of age [F(4,178) = 0.94, P = 0.44].
Collectively, the ED50 values for DMI to decrease immobility time in the TST were lowest in P21 mice, regardless of SERT genotype. Emax values for DMI were greater in P21 mice than in P28 and P90 mice. The role of SERT genotype in antidepressant-like response to DMI was most apparent in P28 mice, in which drug potency decreased with the reduction and loss of SERT, although a similar trend was observed with P21 mice as well (Table 1).
NET Expression Increased with Age in Some Noradrenergic Terminal Regions and Decreased with Age in the Locus Coeruleus
In an effort to explain the age-dependent, antidepressant-like response to DMI, NET binding densities were quantified in limbic (CA1, CA2, CA3, dentate gyrus, prefrontal cortex, and amygdala) and cell body (dorsal raphe and locus coeruleus) regions using quantitative autoradiography to measure specific [3H]nisoxetine binding. Figures 2A and 3A show representative autoradiograms. Figures 2B and 3B are thionine-stained tissue sections to confirm tissue integrity and to highlight brain regions quantified where significant differences were found. These sections are a representative enlargement of the boxed areas shown in the top right panels of Figs. 2A and 3A. Figures 2C and 3C show summary data for specific binding in SERT+/+ mice in the CA3 region of the hippocampus and locus coeruleus, respectively. Bmax and Kd values were analyzed with a two-factor (age, genotype) ANOVA (Fig. 4; Table 2) to determine how age and SERT genotype influence NET binding densities in brain regions of potential importance for antidepressant-like response. These results are described below.
[3H]Nisoxetine Bmax and Kd Values in Terminal Regions.
In the hippocampus, specific [3H]nisoxetine Bmax values significantly increased with age in the CA1 and CA3 regions [F(2,52) = 4.53, P = 0.02; and F(2,52) = 5.40, P < 0.01] but not in the CA2 region of the hippocampus or dentate gyrus [F(2,52) = 2.71, P = 0.08; and F(2,52) = 0.42, P = 0.66] (Fig. 4, A–D). Bmax values in P90 SERT+/+ mice were greater than in P21 SERT+/+ mice in the CA1 and CA3 regions (P = 0.02 and P < 0.01, respectively) (Fig. 4, A and C). Bmax values did not vary as a function of SERT genotype in the CA1, CA2, and CA3 regions of the hippocampus or dentate gyrus [F(4,52) = 1.60, P = 0.19; F(2,52) = 0.21, P = 0.80; F(2,52) = 0.78, P = 0.46; and F(2,52) = 0.87, P = 0.42, respectively]. In addition, no interactions between age and SERT genotype on Bmax values were found in these regions [F(2,52) = 1.76, P = 0.18; F(4,52) = 0.57, P = 0.69; F(4,52) = 0.68, P = 0.61; and F(2,52) = 0.42, P = 0.66, respectively].
Although they were not statistically significant, Bmax values tended to increase with age in the prefrontal cortex and amygdala [F(2,52) = 3.04, P = 0.056; and F(2,52) = 2.32, P = 0.11] (Table 2). Between age group comparisons showed greater Bmax values for P90 SERT+/+ mice than P21 SERT+/+ mice in the prefrontal cortex (P = 0.05), and Bmax values were greater in P90 SERT+/+ mice than P28 SERT+/+ mice in the amygdala (P < 0.01). As for the hippocampus, Bmax values in the prefrontal cortex and amygdala did not vary as a function of SERT genotype [F(2,52) = 1.30, P = 0.28; and F(2,52) = 1.81, P = 0.18, respectively]. No interaction between age and SERT genotype on Bmax values was found in these regions [frontal cortex: F(4,52) = 0.79, P = 0.54; amygdala: F(4,52) = 1.30, P = 0.28]. Within the dorsal raphe, Bmax values were not dependent on either age or SERT genotype [F(2,52) = 0.78, P = 0.46; and F(2,52) = 0.36, P = 0.70] (Table 2). No interaction between age and SERT genotype on Bmax values was found [F(4,52) = 0.58, P = 0.68].
Kd values in all terminal regions ranged from 1.0 to 5.5 nM and were not dependent on age (P ≥ 0.44) or SERT genotype (P ≥ 0.5).
[3H]Nisoxetine Bmax and Kd Values in Cell Body Regions.
Specific [3H]nisoxetine Bmax values decreased with age in the locus coeruleus [F(2,52) = 5.11, P < 0.05] (Fig. 4E). Bmax values were greater in P21 SERT−/− mice than in P90 SERT−/− mice (P = 0.03). Bmax values did not appear to depend on SERT genotype [F(2,52) = 2.53, P = 0.09] and no interaction between age and SERT genotype was found [F(4,52) = 1.00, P = 0.41].
In the locus coeruleus, Kd values ranged from 0.52 to 0.75 nM and did not depend on age (P = 0.67) or SERT genotype (P = 0.25).
Relationship between Maximal Antidepressant-Like Effects of DMI in the TST and Maximal Binding Values for [3H]Nisoxetine in the Hippocampus and Locus Coeruleus
Terminal Regions.
Figure 5, A–D, shows a negative relation between Emax values for DMI’s anti-immobility effects in the TST and Bmax values for specific [3H]nisoxetine binding in the CA1, CA2, and CA3 regions and dentate gyrus of the hippocampus of P21, P28, and P90 SERT+/+, SERT+/−, and SERT−/− mice. In all regions, the relation between Emax and Bmax was not significantly influenced by SERT genotype (P ≥ 0.15); thus, the data were pooled across genotype and were fitted with a single line (Pearson correlation: r = −0.66, −0.74, −0.65, and −0.26 for the CA1, CA2, and CA3 regions and the dentate gyrus, respectively).
Cell Body Regions.
Figure 5E shows a positive relation between Emax values for DMI’s anti-immobility effects in the TST and Bmax values for specific [3H]nisoxetine binding in the locus coeruleus. As for the hippocampus, the relation between Emax and Bmax in the locus coeruleus was not affected by SERT genotype (P = 0.88); data from SERT+/+, SERT+/− and SERT−/− mice were therefore pooled and fitted with a single line (Pearson correlation: r = 0.41).
Discussion
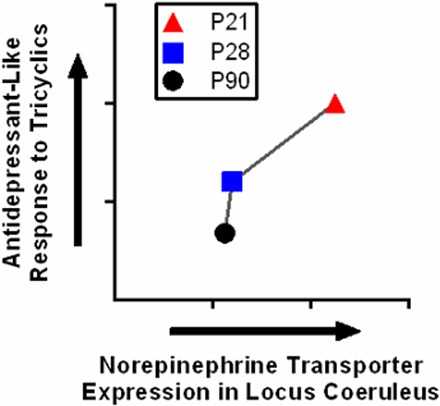
Here, we found the potency and Emax of DMI to be greater in juvenile (P21) and adolescent (P28) SERT+/+ mice than in adult (P90) SERT+/+ mice (Table 1). Regardless of SERT genotype, NET expression, quantified by specific [3H]nisoxetine binding using autoradiography, increased with age in the hippocampus (Fig. 4, A–D) and decreased with age in the locus coeruleus (Fig. 4E). Age-related changes in Emax for DMI to reduce immobility were negatively related with Bmax values for specific [3H]nisoxetine binding in the hippocampus and were positively related with Bmax values in the locus coeruleus (Fig. 5). Thus, there is an inverse relation between the maximal antidepressant-like response after DMI and NET expression in the hippocampus, in which the antidepressant-like response to DMI decreases with age and NET expression increases. In contrast, NET expression in the locus coeruleus and antidepressant-like response to DMI decreases with increasing age. These data reveal a complex relationship between the antidepressant-like response to DMI and NET expression, suggesting that the antidepressant-like effect of DMI may be reliant on brain regions such as the locus coeruleus.
The TCA imipramine, a NET and SERT blocker, is known to reduce immobility time of adolescent mice (P28 and P35) in the FST, an assay of antidepressant-like response (Bourin et al., 1998; David et al., 2001; Mason et al., 2009). Adolescent (P28 and P35) mice also respond to the anti-immobility effects of imipramine and DMI in the TST (Mason et al., 2009; Mitchell et al., 2013), and DMI reduces immobility time in the FST in adolescent (P28 and P30) rats (Pechnick et al., 2008; Reed et al., 2008). Our SERT+/+ mouse data agree with literature showing that adolescents (P28) are sensitive to the antidepressant-like effects of DMI (Mitchell et al., 2013). We found that juvenile (P21) SERT+/+ mice are more sensitive to the antidepressant-like effects of DMI in the TST than P28 mice. These data deviate from experiments using juvenile (P21) rats showing that DMI does not significantly reduce FST immobility (Reed et al., 2008). Possible reasons for this discrepancy include differences in the species (mice versus rats), behavioral assay (TST versus FST) and injection schedule. The FST and TST differ in their sensitivity to detect antidepressant-like activity, which can vary with rodent strain; thus, discrepancies such as this are expected (Cryan et al., 2005).
The SERT-deficient mouse provides a model of SERT gene variants that confer a reduction in SERT expression and/or function and a reduction in SSRI efficacy (Fox et al., 2007; Serretti et al., 2007; Homberg and Lesch, 2011). Our findings are consistent with reports that DMI and imipramine reduce immobility in adult SERT+/+, SERT+/−, and SERT−/− mice in the TST (Holmes et al., 2002; Fig. 1). Studies investigating treatment outcomes of patients with low-expressing SERT gene variants treated with TCAs are limited. Reports using duloxetine (a SERT and NET blocker) and nortriptyline (a NET-selective blocker) have failed to show any association with treatment outcome and low-expressing SERT gene variants (Rajewska-Rager et al., 2008; Perlis et al., 2010). Our results from P28 SERT+/− mice suggest that DMI may have especially limited therapeutic efficacy in adolescents harboring low-expressing SERT gene variants, although the exact mechanism for this remains unknown.
ED50 values increased with age from P21, confirming a similar effect of age in adult (P90) to middle-aged (P300) mice (Mitchell et al., 2015; Table 1). Like DMI potency, Emax values were greatest in SERT+/+ P21 and P28 mice compared with adults (Table 1). These findings are consistent with a study showing a greater antidepressant-like Emax of imipramine in adolescent mice (P28) than in adults using the FST (David et al., 2001). Emax values were not dependent on SERT genotype, which is consistent with studies showing no difference in Emax values between P90 SERT+/+ and P90 SERT+/− or P90 SERT−/− mice (Mitchell et al., 2015).
It should be noted that we cannot rule out other age-dependent factors as modulators of antidepressant-like response (i.e., expression and function of noradrenergic receptors, pharmacokinetic parameters, and expression of NE-synthesizing enzymes) (Murrin et al., 2007). Future studies are needed to better understand the ontogeny of the noradrenergic system and how changes in these parameters may influence the response to psychoactive therapeutics.
The therapeutic benefit of a drug may be affected by its tolerability. Pediatric patients treated with TCAs consistently showed side effects such as vertigo, tremors, low blood pressure, and dry mouth (Hazell and Mirzaie, 2013). Side effects do not necessarily limit antidepressant-like drug effects in the TST. Thus, our findings suggest that TCAs may be efficacious antidepressants in juveniles (lower ED50 and greater Emax), but low tolerability of TCAs may limit the therapeutic benefit of TCAs in pediatric patients. Therefore, our results encourage further investigation of the utility of NET, and/or dual NET and SERT, blockers in the treatment of pediatric depression and the development of new drugs that target transporters for NE and 5-HT but have fewer side effects.
To elucidate the mechanism underlying age-related changes in the antidepressant-like response to DMI, we used quantitative autoradiography to assess [3H]nisoxetine binding to NET (Figs. 2 and 3). Specific [3H]nisoxetine binding densities generally increased with age in noradrenergic terminal regions. Bmax values were greater in adult CA1 and CA3 hippocampal regions than in younger mice (Fig. 4, A and C). Bmax values trended to increase with age in the CA2 region, dentate gyrus, prefrontal cortex, and amygdala (Fig. 4, B and D; Table 1). These findings partially diverge from experiments using [3H]nisoxetine saturation binding with whole hippocampus mouse homogenate, in which no age-related differences in Bmax values among P21, P28, and P90 mice were found (Mitchell et al., 2013). It is conceivable that hippocampal subregion differences in NET expression, which can be revealed by quantitative autoradiography, limited the ability to detect age-related changes in Bmax values for [3H]nisoxetine binding when using hippocampal homogenate preparations. Notably, the Kd values for [3H]nisoxetine here (0.5–5.5 nM) are consistent with Kd values from hippocampal homogenate preparations (2.5–7.4 nM) (Mitchell et al., 2013).
In the rat hippocampus, triphasic [3H]nisoxetine binding patterns from birth through adulthood have been observed (Sanders et al., 2005). Binding densities increased postnatally, peaked between P15 and P25, and decreased into adulthood. In contrast, we found that Bmax values for [3H]nisoxetine binding increased with age from P21 to P90 (CA1 and CA3) or remained unchanged (CA2 and dentate gyrus) (Fig. 4, A–D). In the locus coeruleus of rats, [3H]nisoxetine binding densities were found to peak at P10 and then decrease into adulthood (Sanders et al., 2005). Our data in the mice locus coeruleus are consistent with this finding (Fig. 4E).
It is important to evaluate NET expression in SERT-deficient mice because non-SERT transporters of 5-HT, such as NET, could upregulate to compensate for the loss of SERT (Daws, 2009; Daws et al., 1998, 2005; Baganz et al., 2008). We found that Bmax for [3H]nisoxetine binding was similar among SERT genotypes (Fig. 4), which is consistent with previous findings showing that NET expression remains unchanged in the CA3 region of the hippocampus (Montañez et al., 2003). NET protein levels appear to be uninfluenced by a constitutive reduction or loss of SERT expression.
Increased NET expression in the hippocampus as a function of increasing age correlated with a reduction in the maximal antidepressant-like effect of DMI (Fig. 5, A–D). This relationship was reversed in the locus coeruleus, such that decreasing NET expression as a function of age correlated with a reduction in the maximal antidepressant-like effect for DMI (Fig. 5E). The antidepressant action of TCAs is hypothesized to occur by increasing extracellular levels of NE, primarily via NET blockade in noradrenergic terminal regions such as the hippocampus (Przegaliński et al., 1997; Herr et al., 2012). Given that we found an inverse relationship between NET expression in the hippocampus and antidepressant response (increasing and decreasing, respectively) as a function of age, our data suggest that brain regions other than hippocampus, such as the locus coeruleus, are likely prominent contributors to the antidepressant-like effects of DMI in the TST and warrant further study.
This study evaluated the dose dependence of the antidepressant-like effects of DMI and provided a survey of [3H]nisoxetine binding to brain NET in juvenile, adolescent, and adult SERT wild-type and SERT-deficient mice. Regardless of SERT genotype, DMI potency and Emax were generally greater in juvenile mice than in adults. Of note, P28 SERT+/− and SERT−/− mice were relatively insensitive to the antidepressant-like effects of DMI, suggesting that NET-blocking antidepressants may be especially ineffective in adolescents harboring low-expressing/function variants of SERT, whereas the opposite may be true for juveniles (of any SERT genotype). Regardless of SERT genotype, NET binding increased with age in several noradrenergic terminal regions and decreased with age in the locus coeruleus. These findings help lay the groundwork for future studies investigating the age and SERT genotype dependence of NET-acting drugs, such as reboxetine. Studies to examine the mechanism by which age-dependent variation in NET expression contribute to antidepressant response of NET blockers remain an important avenue for future inquiries. The ultimate goal is to develop effective antidepressants for patients whose symptoms of depression are resistant to treatment with SSRIs.
Acknowledgments
The authors thank Melissa Vitela and Myrna Herrera-Rosales for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- CA
cornu ammonis
- DMI
desipramine
- 5-HT
serotonin
- FST
forced swim test
- NE
norepinephrine
- NET
norepinephrine transporter
- P
postnatal day
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
- TST
tail suspension test
Authorship Contributions
Participated in research design: Mitchell, Gould, Koek, Daws.
Conducted experiments: Mitchell, Bowman, Gould.
Performed data analysis: Mitchell.
Wrote or contributed to the writing of the manuscript: Mitchell, Bowman, Gould, Koek, Daws.
Footnotes
This work was supported by the National Institutes of Health National Institute of Mental Health [Grants MH106978, MH093320, and MH086708] and Congressionally Directed Medical Research Programs [Award AR110109].
References
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, et al. (2008) Organic cation transporter 3: keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci USA 105:18976–18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mössner R, Westphal H, Lesch KP. (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53:649–655. [DOI] [PubMed] [Google Scholar]
- Bourin M, Colombel MC, Redrobe JP, Nizard J, Hascoët M, Baker GB. (1998) Evaluation of efficacies of different classes of antidepressants in the forced swimming test in mice at different ages. Prog Neuropsychopharmacol Biol Psychiatry 22:343–351. [DOI] [PubMed] [Google Scholar]
- Bujoreanu S, Benhayon D, Szigethy E. (2011) Treatment of depression in children and adolescents. Pediatr Ann 40:548–555. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Reed AL. (2007) Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? Neurochem Int 51:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. (1977) Biochemical aspects of neurotransmission in the developing brain. Int Rev Neurobiol 20:65–103. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625. [DOI] [PubMed] [Google Scholar]
- David DJ, Bourin M, Hascoët M, Colombel MC, Baker GB, Jolliet P. (2001) Comparison of antidepressant activity in 4- and 40-week-old male mice in the forced swimming test: involvement of 5-HT1A and 5-HT1B receptors in old mice. Psychopharmacology (Berl) 153:443–449. [DOI] [PubMed] [Google Scholar]
- Daws LC. (2009) Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther 121:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Montañez S, Owens WA, Gould GG, Frazer A, Toney GM, Gerhardt GA. (2005) Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperometry. J Neurosci Methods 143:49–62. [DOI] [PubMed] [Google Scholar]
- Daws LC, Toney GM, Gerhardt GA, Frazer A. (1998) In vivo chronoamperometric measures of extracellular serotonin clearance in rat dorsal hippocampus: contribution of serotonin and norepinephrine transporters. J Pharmacol Exp Ther 286:967–976. [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. (2007) A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 195:147–166. [DOI] [PubMed] [Google Scholar]
- Frazer A. (1997) Pharmacology of antidepressants. J Clin Psychopharmacol 17 (Suppl 1):2S–18S. [DOI] [PubMed] [Google Scholar]
- Hazell P, Mirzaie M. (2013) Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev (6):CD002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell P, O’Connell D, Heathcote D, Robertson J, Henry D. (1995) Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. BMJ 310:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM, Wightman RM. (2012) In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. J Neurophysiol 107:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. (2002) Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology 27:914–923. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. (2011) Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry 69:513–519. [DOI] [PubMed] [Google Scholar]
- Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, Vitela M, Gould GG, Koek W, Daws LC. (2013) Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression. J Neurosci 33:10534–10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. (2001) Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry 49:1002–1014. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. (2008) Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 5:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A, France CP. (2009) Behavioral effects of gamma-hydroxybutyrate, its precursor gamma-butyrolactone, and GABA(B) receptor agonists: time course and differential antagonism by the GABA(B) receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). J Pharmacol Exp Ther 330:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkol RJ, Bendeich EG, Breese GR. (1978) A biochemical and morphological study of the altered growth pattern of central catecholamine neurons following 6-hydroxydopamine. Brain Res 140:125–135. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. (1974) Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol 155:469–481. [DOI] [PubMed] [Google Scholar]
- Loizou LA. (1972) The postnatal ontogeny of monoamine-containing neurones in the central nervous system of the albino rat. Brain Res 40:395–418. [DOI] [PubMed] [Google Scholar]
- Loizou LA, Salt P. (1970) Regional changes in monoamines of the rat brain during postnatal development. Brain Res 20:467–470. [DOI] [PubMed] [Google Scholar]
- Mason SS, Baker KB, Davis KW, Pogorelov VM, Malbari MM, Ritter R, Wray SP, Gerhardt B, Lanthorn TH, Savelieva KV. (2009) Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur J Pharmacol 602:306–315. [DOI] [PubMed] [Google Scholar]
- Mitchell NC, Gould GG, Koek W, Daws LC. (2016) Ontogeny of SERT expression and antidepressant-like response to escitalopram in wild-type and SERT mutant mice. J Pharmacol Exp Ther 358:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NC, Gould GG, Smolik CM, Koek W, Daws LC. (2013) Antidepressant-like drug effects in juvenile and adolescent mice in the tail suspension test: relationship with hippocampal serotonin and norepinephrine transporter expression and function. Front Pharmacol 4:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NC, Koek W, Daws LC. (2015) Antidepressant-like effects and basal immobility depend on age and serotonin transporter genotype. Genes Brain Behav 14:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. (2000) Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res 119:251–257. [DOI] [PubMed] [Google Scholar]
- Montañez S, Owens WA, Gould GG, Murphy DL, Daws LC. (2003) Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem 86:210–219. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Dausse JP, Devynck MA, Meyer P. (1980) Ontogeny of alpha 1 and alpha 2-adrenoceptors in rat brain. Brain Res 190:268–271. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. (2007) Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol 73:1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paksu S, Duran L, Altuntas M, Zengin H, Salis O, Ozsevik SN, Albayrak H, Murat N, Guzel A, Paksu MS. (2014) Amitriptyline overdose in emergency department of university hospital: evaluation of 250 patients. Hum Exp Toxicol 33:980–990. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (1997) The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA. [Google Scholar]
- Pechnick RN, Bresee CJ, Manalo CM, Poland RE. (2008) Comparison of the effects of desmethylimipramine on behavior in the forced swim test in peripubertal and adult rats. Behav Pharmacol 19:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Fijal B, Dharia S, Heinloth AN, Houston JP. (2010) Failure to replicate genetic associations with antidepressant treatment response in duloxetine-treated patients. Biol Psychiatry 67:1110–1113. [DOI] [PubMed] [Google Scholar]
- Przegaliński E, Tatarczyńska E, Dereń-Wesołek A, Chojnacka-Wojcik E. (1997) Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacology 36:31–37. [DOI] [PubMed] [Google Scholar]
- Rajewska-Rager A, Dmitrzak-Weglarz M, Kapelski P, Skibińska M, Kaczmarkiewicz-Fass M, Hauser J. (2008) [Association between polymorphisms of ins/del in the 5-HTT gene and T102C in the 5HTR2A gene and the drug response for escitalopram and nortriptyline in depressed patients]. Psychiatr Pol 42:903–914. [PubMed] [Google Scholar]
- Reed AL, Happe HK, Petty F, Bylund DB. (2008) Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacology (Berl) 197:433–441. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. (2005) Development of the norepinephrine transporter in the rat CNS. Neuroscience 130:107–117. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. (2007) Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 12:247–257. [DOI] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM. (1992) [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther 260:427–436. [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. (1995) Noradrenaline is essential for mouse fetal development. Nature 374:643–646. [DOI] [PubMed] [Google Scholar]