FIGURE 14.
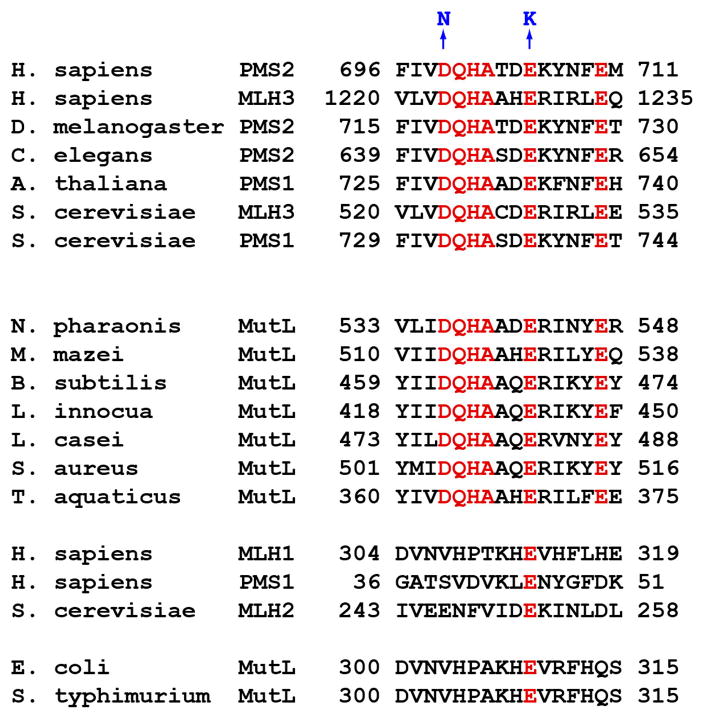
MutLα endonuclease active site motif.
C-terminal PMS2 DQHA(X)2E(X)4E endonuclease active site motif is conserved in eukaryotic PMS2 homologs (S. cerevisiae PMS1 is a homolog of human PMS2) and in many bacterial MutL proteins, with the exception of MutL proteins from bacteria like E. coli that rely on d(GATC) methylation to direct mismatch repair. Amino acid residues shown in blue at the top of the figure correspond to substitution mutations used to assess involvement of the motif in MutLα function.
The figure is reproduced with from reference 75, Copyright 2006 with permission from Elsevier.