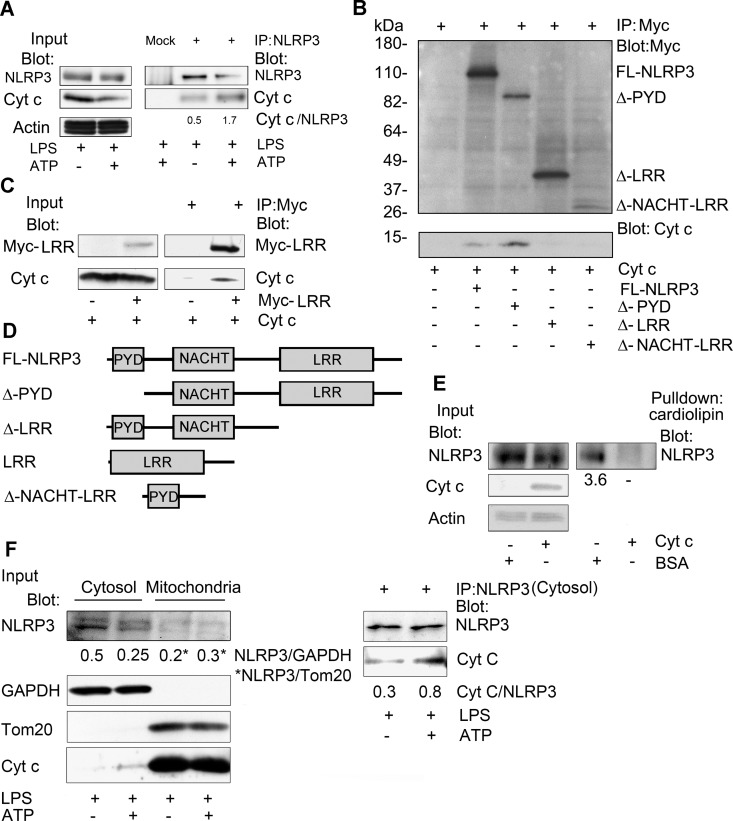
Fig 2. Cytochrome c binds to the LRR repeats of NLRP3 and interferes with cardiolipin binding.
(A) Immunoblots of cell lysates and either NLRP3 or sham immunoprecipitates prepared from LPS-primed THP-1 macrophage cells, stimulated with ATP or not, to detect an interaction between endogenous NLRP3 and cytochrome c. The ratios between the NLRP3 and cytochrome c levels in the immunoprecipitates were quantitated using Image J. (B) Immunoblots of cell lysates and either control or Myc immunoprecipitates to map the portion of NLRP3 important for the interaction with cytochrome c. Myc-tagged NLRP3 constructs were transfected into HEK 293T cells. The immunoprecipitates were washed, incubated with cytochrome c (50 ng), washed again, fractionated by SDS-PAGE, and immunoblotted. (C) Immunoblots of cell lysates and Myc-LRR domain immunoprecipitates incubated with cytochrome c, or not. A construct expressing a myc tagged NLRP3 LRR domain was transfected into HEK 293T cells. The Myc and control immunoprecipitates were incubated with cytochrome c (50 ng), washed, and immunoblotted. (D) Schematic of the constructs used in the above experiments (B & C). (E) Immunoblots of cell lysates and cardiolipin bead pull-downs to assess whether cytochrome c interferes with the interaction between cardiolipin and NLRP3. BSA (50 ng) or purified cytochrome c (50 ng) was added to lysates prepared from HEK 293T cells expressing NLRP3-Flag. Following a 30 minute incubation cardiolipin conjugated beads were added to the lysates for an additional 30 minutes. The caridolipin beads were washed; and the bound NLRP3 eluted in SDS-sample buffer, size fractionated by SDS PAGE, and quantitated by immunoblotting. The amount of NLRP3 in the cardiolipin pulldowns was normalized to the BSA control. The above experiments were respectively performed twice. (F) Cell lysates from cell treated with ATP, or not, were fractionated into cytosolic and mitochondrial fractions and the indicated proteins were immunoblotted. NLRP3 immunoprecipitates were prepared using the cytosolic fraction. An interaction between endogenous NLRP3 and cytochrome c was assess by immunoblotting. The ratios between the NLRP3 and cytochrome c levels in the immunoprecipitates were quantitated using Image J.