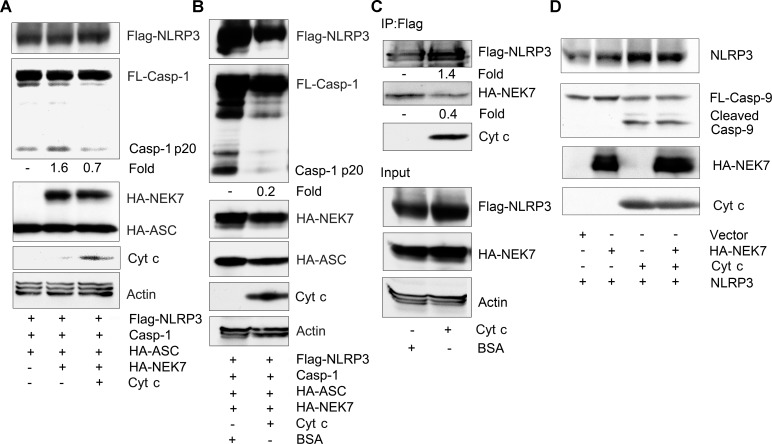
Fig 5. Cytochrome c reduces NEK7 mediated NLRP3 inflammasome activation and the interaction between NEK7 and NLRP3.
(A) Immunoblots of cell lysates from HEK 293T cells transfected with constructs expressing FLAG-NLRP3, caspase-1, and HA-ASC in the presence or absence of HA-NEK7, and the presence or absences of construct expressing cytochrome c to assess caspase-1 cleavage. (B) Immunoblots of cell lysate from Flag-NLRP3, caspase-1, and HA-ASC transfected HEK 293T cells mixed with cell lysates from HA-NEK7 transfected HEK 293T cells and incubated with either BSA or cytochrome c to assess caspase-1 cleavage. The mixed cells lysates were incubated for 30 minutes at 30°C prior to immunoblotting for the indicated proteins. (C) Immunoblots of cell lysates and Flag-NLRP3 immunoprecipitates mixed with HA-NEK7 expressing cell lysates to assess whether cytochrome c affects the interaction between NLRP3 and NEK7. Cell lysates from HEK 293T cells expressing HA-NEK7 were added to Flag-NLRP3 immunoprecipitates and incubated with BSA or cytochrome c for 1 hour at room temperature. After which the anti-Flag beads were washed, the bound proteins eluted, and used for immunoblotting. Input levels in the cell lysates are also shown. (D) Immunoblots of a mixture of cell lysates prepared from LPS stimulated differentiated THP-1 cells and HEK 293T cell expressing HA-NEK7 to assess caspase-9 cleavage. The mixed cell lysates were incubated with cytochrome c (0.25 mg/ml) for 30 minutes at 30°C, or not. The above experiments were respectively performed twice.