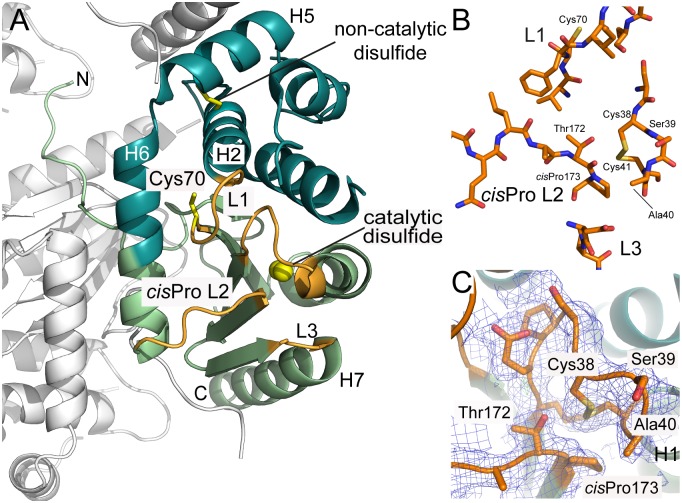
Fig 4. Crystal structure of CtDsbA.
A. The crystal structure of CtDsbA contains a thioredoxin domain (light green) and a helical domain (dark green.) Loops on the catalytic surface that constitute the active site of CtDsbA and determine redox activity are colored orange and labeled. The active site catalytic disulfide is highlighted with sulfurs shown as yellow spheres. The non-catalytic disulfide (between Cys84 and Cys145) and the single thiol (Cys70) in L1 are shown in stick representation. The most N-terminal region of CtDsbA is unstructured. Crystal packing interactions with the second monomer in the asymmetric unit and a symmetry related molecule (shown in white) stabilize this region of the protein such that is well resolved in the electron density map. B. Close view of the four loops (L1, cisPro L2, L3 and the Cys-Ser-Ala-Cys motif) which constitute the active site surface of CtDsbA. C. In the crystal structure the active site cysteines are oxidized. Analysis of bond distances indicates that the Cys 38 thiolate could be stabilized by favorable bond interactions with Thr 172 (3.4 Å between Thr 172 OH and Cys 38 SG in the oxidized structure) of the neighboring cisPro L2 consistent with an oxidizing protein character. The Cys 41 sulfur atom is 3.5 Å from the Thr 172 hydroxyl in the oxidized structure. 2Fo-Fc and Fo-Fc electron density maps for the active site and cisPro Loop 2 were generated from calculated phases using phenix.maps and are shown contoured at 1.0 σ and 3.0 σ respectively. The maps are shown within a 1 Å radius of each atom of each loop.